The spectral lines of hydrogen as shown in Figure are grouped in separate series. In each series, the distance of separation between the
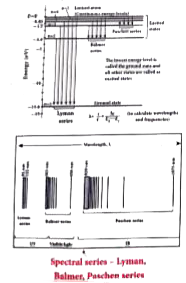
consecutive wavelengths decreases from higher wavelength to the lower wavelength, and also wavelength in each series approach a limiting value known as the series limit. The wavelengths of these spectral lines perfectly agree with the equation derived from Bohr atom model.
`1/lamda=R(1/n^(2)-1/m^(2))=barv" "...(1)`
where `barv` is known as wave number which is inverse of wavelength, R is known as Rydberg constant.
(a) Lyman series :
Put n = 1 and m = 2,3,4..... in equation (1). The wave number or wavelength of spectral lines of Lyman series which lies in ultra-violet region is
`barv=1/lamda=R(1/1^(2)-1/m^(2))`
(b) Balmer series :
Put n = 2 and m = 3,4,5..... in equation (1). The wave number or wavelength of spectral lines of Balmer series which lies in visible region is
`barv=1/lamda=R(1/2^(2)-1/m^(2))`
( c) Paschen series :
Put n = 3 and m = 4,5,6..... in equation (1). The wave number or wavelength of spectral lines of Paschen series which lies in infra-red region (near IR) is
`barv=1/lamda=R(1/3^(2)-1/m^(2))`
(d) Brackett series :
Put n = 4 and m = 5,6,7 ....... in equation (1). The wave number or wavelength of spectral lines of Brackett series which lies in infra - red region (middle IR) is
`barv=1/lamda=R(1/4^(2)-1/m^(2))`
(e) P fund series :
Put n = 5 and m = 6,7,8..... in equation (1). The wave number or wavelength of spectral lines of P fund series which lies in infra-red region (far IR) is
`barv=1/lamda=R(1/5^(2)-1/m^(2))`
