Davisson - Germer experiment :
(i) De Broglie hypothesis of matter waves was experimentally confirmed by Clinton Davisson and Lester Germer. (ii) They demonstrated that electron beams are diffracted when they fall on crystalline solids. Since crystal can act as a three dimensional diffraction grating for matter waves, the electron waves incident on crystals are diffracted off in certain specific directions.
(iii) The filament F is heated by a low tension (L.T.) battery. Electrons are emitted from the hot filament by thermionic emission. (iv) They are then accelerated due to the potential difference between the filament and the anode aluminium cylinder by a high tension (H.T.) battery. (v) Electron beam is collimated by using two thin aluminium diaphragms and is allowed to strike a single crystal of Nickel. (vi) The electrons scattered by Ni atoms in different directions are received by the electron detector which measures the intensity of scattered electron beam.
(vii) The detector is rotatable in the plane of the paper so that the angle `phi` between the incident beam and the scattered beam can be changed at our will. The intensity of the scattered electron beam is measured as a function of the angle `theta`.
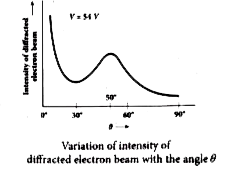
(viii) Figure shows the variation of intensity of the scattered electrons with the angle `theta` for the accelerating voltage of 54V. For a given accelerating voltage V, the scattered wave shows a peak or maximum at an angle of `50^@` to the incident electron beam.
(ix) This peak in intensity is attributed to the constructive interference of electrons diffracted from various atomic layers of the target material.
(x) From the known value of interplanar spacing of Nickel, the wavelength of the electron wave has been experimentally calculated as 1.65 Å.
(xi) The wavelength can also be calculated from de Broglie relation for V = 54 V from equation as
`lamda = (12.27)/sqrtV""Å = (12.27)/sqrt(54)`
`lamda = 1.67 Å`
(xii) This value agrees well with the experimentally observed wavelengti of 1.65Å. Thus this experiment directly verifies de Broglie.s hypothesis of the wave nature of moving particles.
