Similar Questions
Explore conceptually related problems
Recommended Questions
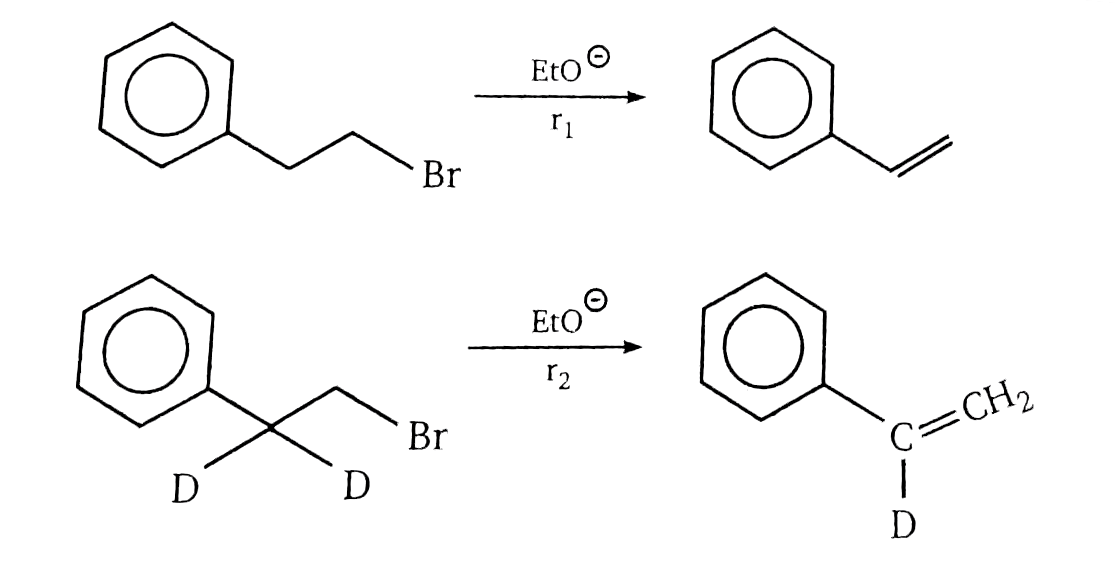
- Compare rate of reactions.
Text Solution
|
- Conisder a gaseous reaction, the rate of which is given by k[A][B]. Th...
Text Solution
|
- Compare the rate of reaction of following species with OH^(-) : (i)
Text Solution
|
- Compare the rate of reaction of following for nitration reaction.
Text Solution
|
- Compare rate of reactions.
Text Solution
|
- The rate law for a certain reaction is found to be : Rate = k[A] [B]^(...
Text Solution
|
- Compare the rate of reaction:-
Text Solution
|
- The rate constant of two reactions at 30^(@)C are equal. The temperatu...
Text Solution
|
- For every 10 K rise in temperature , the rate of a reaction becomes d...
Text Solution
|