Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
SUBHASH PUBLICATION|Exercise THREE MARKS QUESTIONS|28 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
SUBHASH PUBLICATION|Exercise THREE MARKS QUESTIONS|28 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2019
SUBHASH PUBLICATION|Exercise PART-E|7 VideosCHEMICAL EQUILIBRIUM
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS|34 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-TWO MARK QUESTIONS AND ANSWERS
- Why do C show 4 valency?
Text Solution
|
- Draw the sp^(3) hybridized C.
Text Solution
|
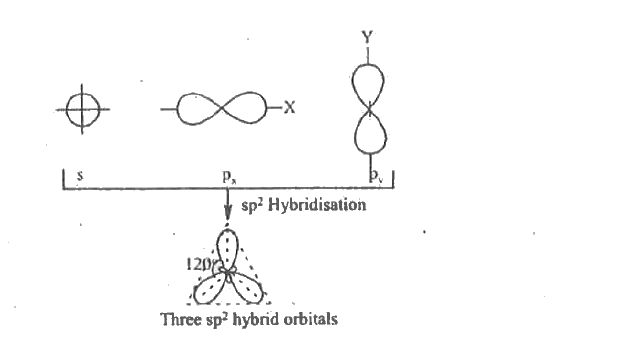
- Draw the sp^(2) hybridized C.
Text Solution
|
- Draw the sp^ hybridized C.
Text Solution
|
- Explain ionic bond in NaCl.
Text Solution
|
- Meation the condition favouring ionic bond formation.
Text Solution
|
- Mention any two characteristies of ionic compounds.
Text Solution
|
- Mention the factors fovauring covanlent bond.
Text Solution
|
- Mention the characteristic of covalent molecule.
Text Solution
|
- Explain an example for covalent bond.
Text Solution
|
- What do you mean by Co-ordinate Bond ? Explain with example.
Text Solution
|
- HCl form polar bond.
Text Solution
|
- Ionic solids do not conduct.
Text Solution
|
- NH(3) polar but BF(3) non-polar.
Text Solution
|
- HF is liquid where as HCl, HBr …… Other hydrogen halide is liquid.
Text Solution
|
- O-Nitrophenol is more volatile than p-Nitrophenot (Or) boling point of...
Text Solution
|
- Covalent compounds have low melting point.
Text Solution
|
- H(2)O is liquid whereas H(2)S is gas.
Text Solution
|
- C-II bond is polar but CH(4) is non polar.
Text Solution
|
- Density of water is maximum at 4^(@)C.
Text Solution
|