Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
SUBHASH PUBLICATION|Exercise THREE MARKS QUESTIONS|28 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
SUBHASH PUBLICATION|Exercise THREE MARKS QUESTIONS|28 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2019
SUBHASH PUBLICATION|Exercise PART-E|7 VideosCHEMICAL EQUILIBRIUM
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS|34 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-TWO MARK QUESTIONS AND ANSWERS
- Water has high boiling point.
Text Solution
|
- Ice floats on water or ice is lighter than water.
Text Solution
|
- CO(2)andC(2)H(2) (acetylene) are non-polar.
Text Solution
|
- sigma bond is stronger compare to p bond.
Text Solution
|
- Ethyl alcohol is an organic compound but still freely soluble in water...
Text Solution
|
- Explain covalent bond in carbonadioxide.
Text Solution
|
- Explain covalent in acetylene.
Text Solution
|
- Write the lewis do structure for H(2) and oxygen molecule.
Text Solution
|
- Write the lewis dot structure of CO(3)^(2-) molecule.
Text Solution
|
- Explain the polarity in H(2)O molecule.
Text Solution
|
- Explain the polarity of Borontrifluoride molecule.
Text Solution
|
- Write the formation of sigma covalent bond diagrammatically.
Text Solution
|
- Wirte the formation of pi bond (p) diagrammatically.
Text Solution
|
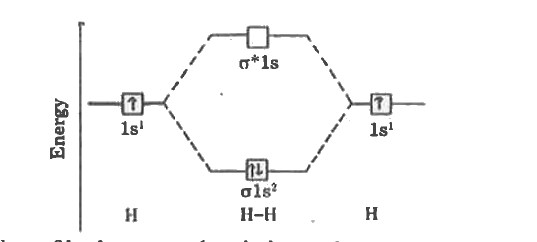
- Write the energy level diagram of hydrogen molecule.
Text Solution
|
- Draw energy level diagram for He-2 molecule.Calculate its bond order.
Text Solution
|
- Give two difference between bonding molecular orbital and antibonding ...
Text Solution
|
- Write the resonance structures of ozone.
Text Solution
|
- Write the resonance structure of CO(3)^(2-)
Text Solution
|
- Explain resonance concept.
Text Solution
|
- Wirte the energy level diagram of lithium molecule.
Text Solution
|