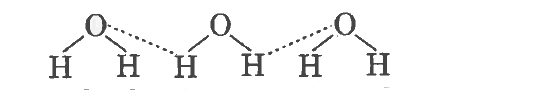Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-THREE MARKS QUESTIONS
- Based on VSEPR theory explain the structure of ammonia.
Text Solution
|
- Based on VSERP theor explain the structure of water.
Text Solution
|
- Whate are polar and None Polar convalent bond explain with example.
Text Solution
|
- Define dipole moment ? Comment on structure & dipole moment of CO(2),...
Text Solution
|
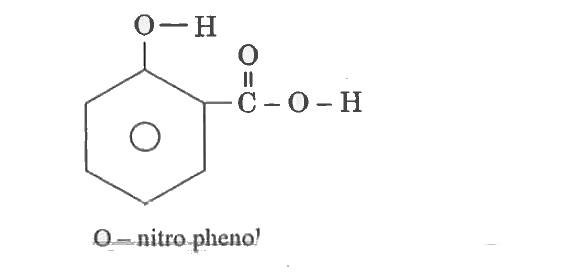
- Define Hydrogen Bond ? Explain its type with example.
Text Solution
|
- Explain Valence Bond Theory.
Text Solution
|
- Salient features of Molecules Orbital Theory (MOT)
Text Solution
|
- Calculate the formal charge on each oxygen atom in ozone.
Text Solution
|
- discuss the limitation of ocet rule.
Text Solution
|
- Compare the polarity nature of NH(3) and NF(3).
Text Solution
|
- Discuss Fajan's rules ?
Text Solution
|
- Based on VSEPR theory explain the structure of methane.
Text Solution
|
- Explain sp^(3)d^(2) hybridization SF(6) molecule.
Text Solution
|
- Explain sp^(3)d hybridization PCl(5) molecule.
Text Solution
|
- Write the salient features of hybridisation. S
Text Solution
|
- Explain the type of hybridization.
Text Solution
|
- Explain the formation of molecule orbital by LCAO method.
Text Solution
|
- Mention the conditions for combination of atomic orbitals.
Text Solution
|
- Draw the energy level diagramn of carbon molecule.Calculate its bond o...
Text Solution
|
- Write the energy level diagram of oxygen molecule.
Text Solution
|