Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-REDOX REACTIONS-Three Marks Questions
- Explain oxidation and reduction in terms of loss and gain of electrons...
Text Solution
|
- Balance the following redox reaction by half reaction method. Fe^(2...
Text Solution
|
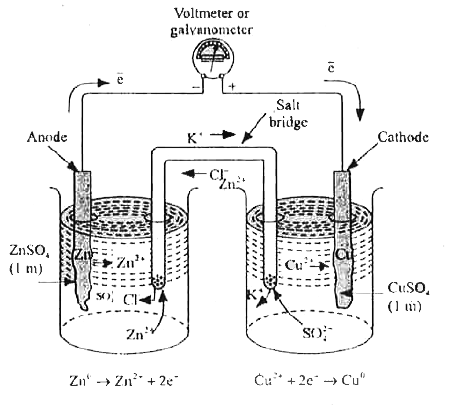
- Explain the redox reaction in galvanic cells.
Text Solution
|
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- Mention which gets reduced and which gets oxidized in the following re...
Text Solution
|
- Mention which gets reduced and which gets oxidized in the following re...
Text Solution
|
- Identify the oxidant and reductant in the following reactions: 10H^(...
Text Solution
|
- Identify the oxidant and reductant in the following reactions: I2(g)...
Text Solution
|
- Write the rules for assigning oxidation number.
Text Solution
|
- Find the oxidation state of sulphur in the following compounds: H2S,H2...
Text Solution
|
- Find the oxidation number of the element underlined in the following s...
Text Solution
|
- Find the oxidation number of the element underlined in the following s...
Text Solution
|
- What is the oxidation number of the metals [Fe(CN)6]^(4-)
Text Solution
|
- What is the oxidation number of Mn is MnO(4)^(-)?
Text Solution
|
- Explain applications of Redox Reactions?
Text Solution
|
- A cell is prepared by dipping a chromium rod is 1 M Cr2(SO4)3 solution...
Text Solution
|
- Permanganate ion reacts with bromide ion is a basic medium, to give ma...
Text Solution
|
- Permangante (VII) ion, in basic solution oxidizes iodide ion I- to pro...
Text Solution
|
- What is the oxidation number of sulphur in the following molecules/ io...
Text Solution
|
- What is the oxidation number of sulphur in the following molecules/ io...
Text Solution
|