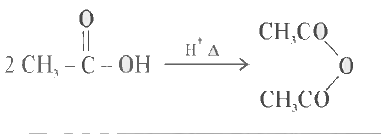Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS-QUESTIONS
- Carboxylic acids are more acidic than phenols. Give reason.
Text Solution
|
- Explain the effect of electron withdrawing groups. [EWG] on the acidi...
Text Solution
|
- What is the effect of electron withdrawing group on the acidity of car...
Text Solution
|
- Explain the effect of electron donating group (EDG) on the acidity of ...
Text Solution
|
- Among formic acid and acetic acid, which is more acidic ? Give reason...
Text Solution
|
- How PCl(3), PCl(5) and SOCl(2) reacts with carboxylic acids ?
Text Solution
|
- Write the chemical equation to convert ethanoic acid to ethanoic anhyd...
Text Solution
|
- The equation for The reaction between carboxylic acdis and PCl(5)
Text Solution
|
- Give the equation for The reaction between formaldehyde and con. K...
Text Solution
|
- Give the equation for The formation of oxime from carbonyl compound...
Text Solution
|
- A carboxylic acid is heate with alcohol in presence of con. H(2)SO(4)....
Text Solution
|
- Complete the equation.
Text Solution
|
- How carboxylic acids react with ammonia ?
Text Solution
|
- How do you convert benzoic acid to benzamide ? Write the equation.
Text Solution
|
- What is the major product of the following reaction.
Text Solution
|
- Explain decarboxylation reaction with an example.
Text Solution
|
- What is soda lime ?
Text Solution
|
- Explain HVZ reaction with example.
Text Solution
|
- Complete the reaction
Text Solution
|
- Complete the equation CH(3)COONa overset(NaOH+CaO)underset(Delta)to
Text Solution
|