Text Solution
Verified by Experts
Topper's Solved these Questions
ANNUAL EXAMINATION QUESTION PAPER MARCH 2019
SUBHASH PUBLICATION|Exercise PART D|11 VideosANNUAL EXAMINATION QUESTION PAPER MARCH 2019
SUBHASH PUBLICATION|Exercise PART B|8 VideosANNUAL EXAMINATION QUESTION PAPER MARCH 2018
SUBHASH PUBLICATION|Exercise PART-D|24 VideosBIOMOLECULES
SUBHASH PUBLICATION|Exercise QUESTION|77 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER MARCH 2019-PART C
- (a) In extraction of Aluminium by electrolysis, (i) Write ovreall ce...
Text Solution
|
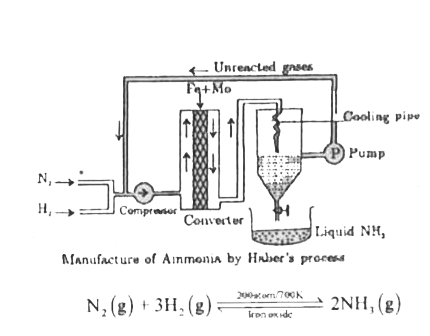
- In the manufacture of ammonia by Haber's process. Write the flow chart...
Text Solution
|
- (a) Give reason: (i) Hydrogen bonding in H(2)O but not in H(2)S. (ii...
Text Solution
|
- Complete the following chemical equations : (i) NH3 + 3CI2 to … + 3H...
Text Solution
|
- Write the balanced chemical equation involved in the manufacture of po...
Text Solution
|
- (i) What are interstial compounds? (ii) Transition metals show good ca...
Text Solution
|
- a) write the IUPAC name of K3 [Cr (C2 O4)3]. b) give the facial (fac...
Text Solution
|
- Explain the hybridisation, geometry and magnetic property of [Ni(Cl)(4...
Text Solution
|