Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER MARCH 2019-PART D
- (a) Calculate the number of particles in Face Centred Cubic (FCC) latt...
Text Solution
|
- (a) 31 g of an unknown molecular material is dissolved in 500 g of wat...
Text Solution
|
- (a) Write the equations for the reactions taking place at anode and ca...
Text Solution
|
- (a) Derive an integrated rate equation for the rate constant of a firs...
Text Solution
|
- Answer any three of the following questions. a. Define Shape Selecti...
Text Solution
|
- (a) Write the equations for the steps involved in the S(N)1 mechanism ...
Text Solution
|
- (a) Explain the mechanism of acid catalysed dehydration of ethanol to ...
Text Solution
|
- (a) How does benzene reacts with acetyl- chloride in the presence of a...
Text Solution
|
- (a)Between CH(3)NH(2)and C(6)H(5)NH(2) which is more base? Give reason...
Text Solution
|
- (a) Write the Haworth structure of maltose. (b) What is peptide Linkag...
Text Solution
|
- (a) How is Buna-N prepared? Give equation (b) Name the monomers of Nyl...
Text Solution
|
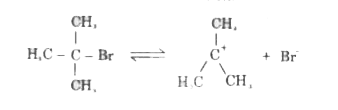
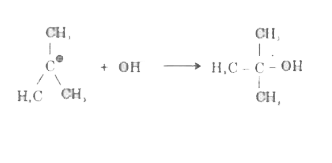 .
.