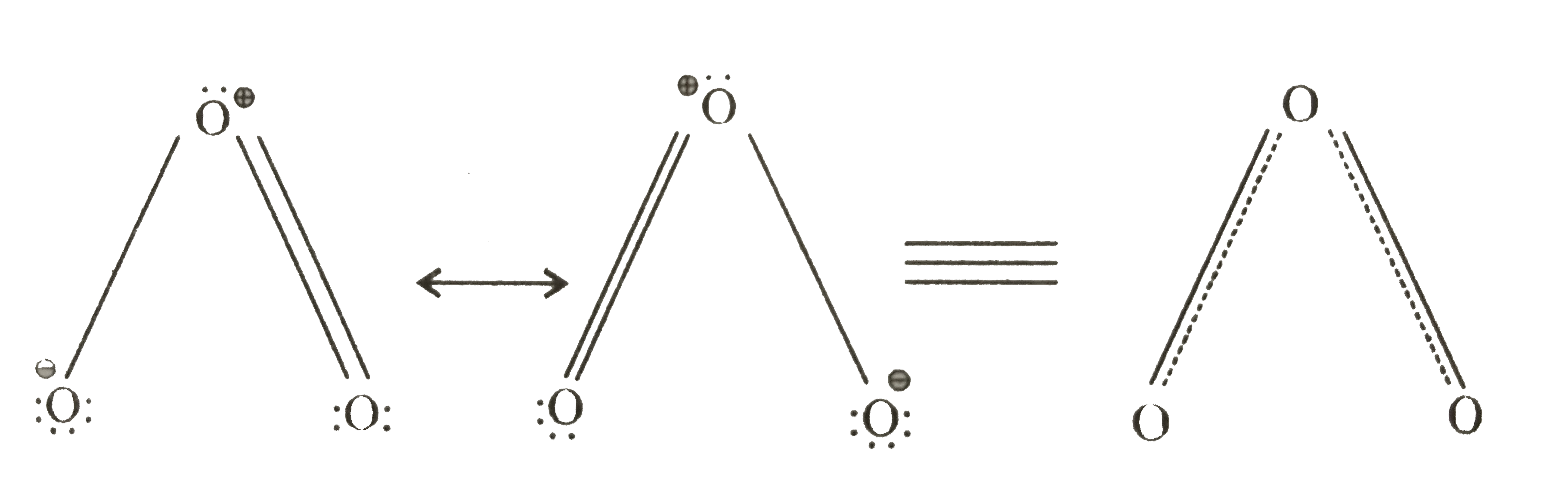
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-SET-III
- Name the principal ore of aluminium. Explain the significance of leach...
Text Solution
|
- Define the following terms with an example in each case: (i) Macromo...
Text Solution
|
- Give reasons for the following : (i) Though nitrogen exhibits + 5 ox...
Text Solution
|
- Write the main products of the following reactions:
Text Solution
|
- Out of , which is an example of a benzylic halide?
Text Solution
|
- Write the formula of the compound of iodine which is obtained when con...
Text Solution
|
- What type of colloid is formed when a gas is dispesed in a liquid? Giv...
Text Solution
|
- Write he IUPAC name of the following compound: CH(3)-O-underset(CH(3...
Text Solution
|
- Draw the structure of te following: (a) XeF(4) (b) BrF(4)
Text Solution
|
- Write the anme of the cell which is generally used in transistors. Wri...
Text Solution
|
- Using IUPAC norms write the formulae for the following: (a) Potassiu...
Text Solution
|
- (a) What type of isomerism is shown by the complex [Co(NH(3))(5)(SCN)]...
Text Solution
|
- (a) Based on the nature of intermolecular forces, classify the followi...
Text Solution
|
- (a) Write the principle of electrolytic refining. (b) Why does coppe...
Text Solution
|
- Define the following: (a) Cationic detergents (b) Broad spectrum a...
Text Solution
|
- Write the structures of the monomers used for getting the following po...
Text Solution
|
- Define " Order of a reaction" and " Activation energy of a reaction "...
Text Solution
|
- What is meant by reverse osmosis?.
Text Solution
|
- What type of ores can be concentrated by magnetic separation method ...
Text Solution
|
- Which compounds used to estimate the hardness of water volumetrically ...
Text Solution
|