Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-CHEMISTRY (THEORY)
- An element crystallizes in b.c.c. lattice with cell edge of 500 pm. Th...
Text Solution
|
- For the first order thermal decomposition reaction, the following data...
Text Solution
|
- Define the following terms : (i) Lyophilic coloid (ii) Zeta poten...
Text Solution
|
- (i) Name the method of refining of nickel (ii) What is the role of c...
Text Solution
|
- Calculate the boiling point of solution when 4g of Mg SO(4) (M=120g "m...
Text Solution
|
- Give reasons : (i) SO(2) is reducing while TeO(2) is an oxidizing agen...
Text Solution
|
- Write the final product(s) in each of the following reactions : (a)...
Text Solution
|
- Account for the following (i)Primary amines (R-NH(2)) have higher b...
Text Solution
|
- How do you convert: 1. Chlorobenzene to biphenyl (ii). Propene to ...
Text Solution
|
- Write the major product(s) in the following : (i) (ii) 2CH(3)-...
Text Solution
|
- (i) Whate is the role of Sulphur in the vulcanization of rubber ? (...
Text Solution
|
- (i) Write the structural difference between starch and cellulose. (i...
Text Solution
|
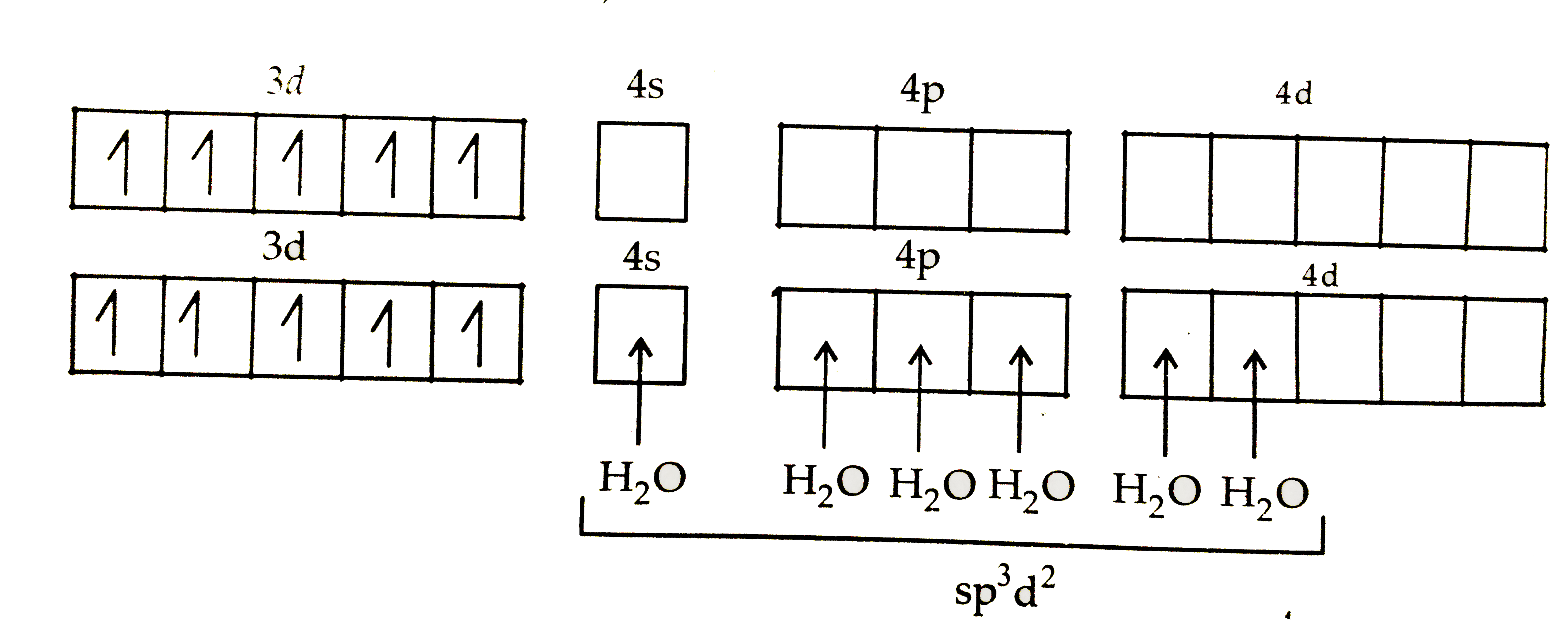
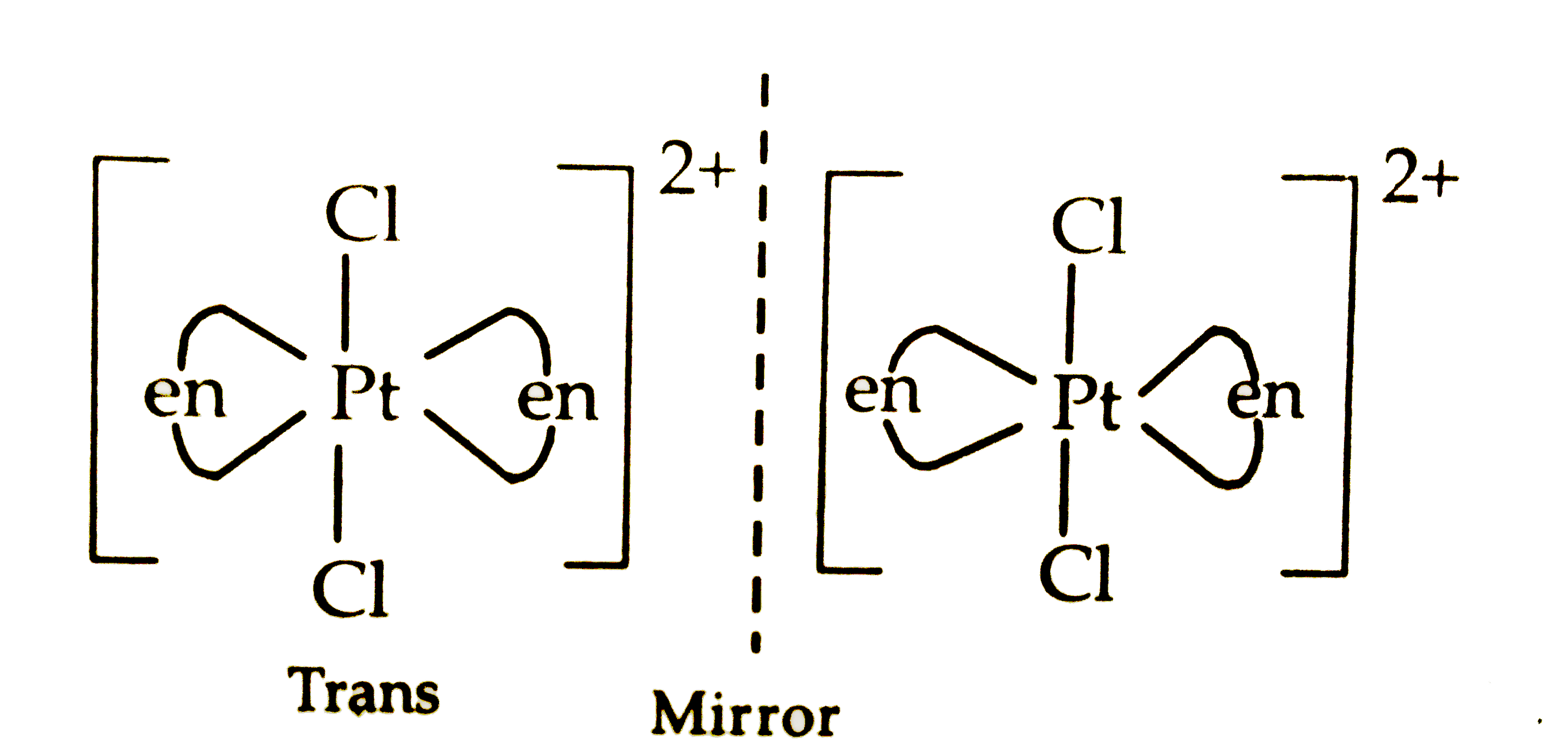
- (a) For the complex [Fe(H(2)O)(6)]^(3+) , write the hybridization, mag...
Text Solution
|
- Due to hectic and busy schedule, Mr. Singh started taking junk food in...
Text Solution
|
- Calculate E(cell)^(@) for the following reaction at 298 K: 2Al(s) +...
Text Solution
|
- The conductivity of 0.001 "mol" L^(-1) solution of CH(3)COOH " is " 3....
Text Solution
|
- (a) Account for the following : (i) Mn shows the highest oxidation s...
Text Solution
|
- The elements of 3d transition series are given as : Sc Ti V ...
Text Solution
|
- (a) Write the structures of A and B in the following reactions : (i...
Text Solution
|
- (a) Write the chemical reaction involved in Wolf-Kishner reduction. ...
Text Solution
|