A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
JEE ADVANCED 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION-3|6 VideosJEE ADVANCED 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION-3|6 VideosJEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise PHYSICS SECTION - IV Matrix Match Type|2 VideosJEE ADVANCED 2021
JEE ADVANCED PREVIOUS YEAR|Exercise QUESTION|38 Videos
JEE ADVANCED PREVIOUS YEAR-JEE ADVANCED 2020-SECTION-2
- A particle of mass m moves in circular orbits with potential energy N(...
Text Solution
|
- The filament of a light bulb has surface has area 64mm^(2). The filame...
Text Solution
|
- Some times it is convenient to construct a system of units so that qua...
Text Solution
|
- A uniform electric field vec(e )=-4000sqrt(3)hat(y)NC^(-1) is applied ...
Text Solution
|
- Shown in the figure is a semi - circular metallic strip that has thick...
Text Solution
|
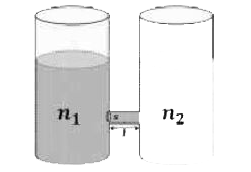
- As shown schematically in the figure , two vessels contain water solut...
Text Solution
|