Text Solution
Verified by Experts
The correct Answer is:
JEE ADVANCED PREVIOUS YEAR-JEE (ADVANCE) 2020-SECTION 3
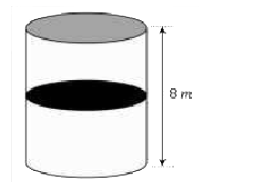
- A thermally isolated cylindrical closed vessel of height 8 m is k...
Text Solution
|
- A spherical bubbles inside water has radius R . Take the pressuere i...
Text Solution
|
- In the balanced condition , the values of the resistance of the foura...
Text Solution
|
- Two capacitor with capacitance values C1 = 2000 om 10 pF and C2 = 3000...
Text Solution
|
- A cubical solid aluminium (bulk modulus = -V (dp)/(dV) = 70 Gpa) block...
Text Solution
|
- The inductors of the two LR circuits are placed next to each other , a...
Text Solution
|
- A container with 1 kg of water in it kept in sunlight , which causes t...
Text Solution
|