A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
JEE (ADVANCED ) 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION -III|6 VideosJEE (ADVANCED ) 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION -III|6 VideosMOCK TEST 2022
JEE ADVANCED PREVIOUS YEAR|Exercise QUESTIONS|14 VideosJEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise CHEMISTRY SECTION - IV : Matric Match Type|2 Videos
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR-JEE (ADVANCED ) 2020-SECTION -II
- In thermodynamics, the P-V work done is given by w=-intdVP("ext.") ...
Text Solution
|
- With respect to the compounds I-V, choose the correct statement(s).
Text Solution
|
- In the reaction scheme shown below Q, R, and S are the major products....
Text Solution
|
- Choose the correct statement(s) among the following:
Text Solution
|
- With respect to hypochlorite, chlorate and perchlorate ions, choose th...
Text Solution
|
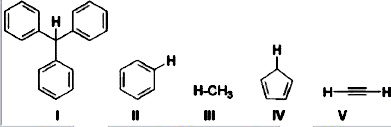
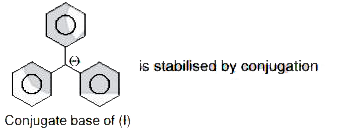
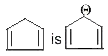 which is an aromatic anion
which is an aromatic anion