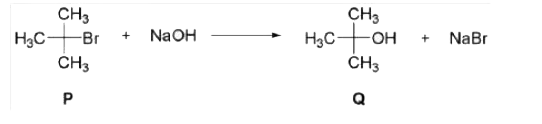
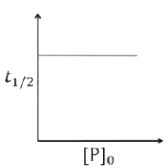
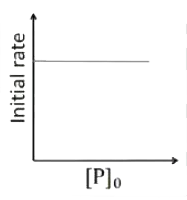
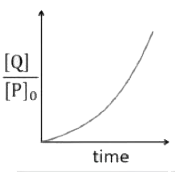
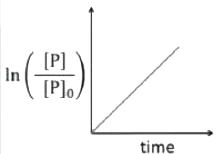
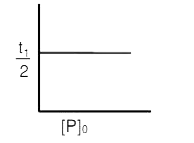
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
JEE ADVANCED 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION-3|6 VideosJEE ADVANCED 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION-3|6 VideosJEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise CHEMISTRY SECTION - IV : Matric Match Type|2 VideosJEE ADVANCED 2021
JEE ADVANCED PREVIOUS YEAR|Exercise QUESTION|38 Videos
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR-JEE ADVANCED 2020-SECTION-2
- In an experiment, grams of a compound X (gas/liquid/solid)taken in a c...
Text Solution
|
- Which of the following plots is(are) correct for the given reaction? (...
Text Solution
|
- Which among the following statement(s) is (are) true for the extractio...
Text Solution
|
- Choose the correct statement(s) among the following.
Text Solution
|
- Consider the following four compounds I, II, III, and IV. Choose ...
Text Solution
|
- Consider the following transformations of a compound P. Choose th...
Text Solution
|