Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR-JEE ADVANCED 2020-SECTION-3
- A solution of 0 .1 weak base (B )is titrated with 0.1 M of a strong a...
Text Solution
|
- Liquids A and B from ideal solution for all composition of A and B at ...
Text Solution
|
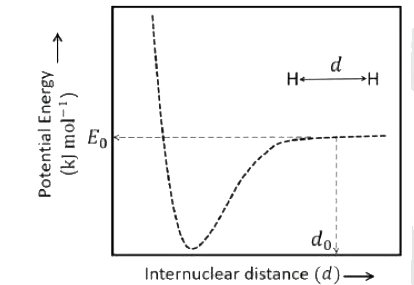
- The figure is the plot potential energy versus internuclear distance (...
Text Solution
|
- Consider the reaction sequence from P to Q shown below . The overall y...
Text Solution
|
- Tin is obtained from cassiterite by reduction with coke. Use the data ...
Text Solution
|
- An acidified of 0.05 M Zn^(2+) is saturated with 0.1 M H2S. What is th...
Text Solution
|