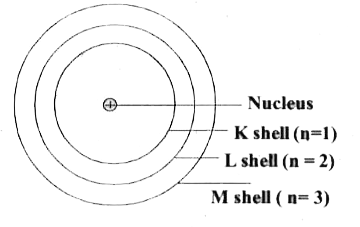
Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF THE ATOM
CPC CAMBRIDGE PUBLICATION|Exercise ADDITIONAL QUESTIONS (I. Choose the correct answer)|10 VideosSTRUCTURE OF THE ATOM
CPC CAMBRIDGE PUBLICATION|Exercise ADDITIONAL QUESTIONS (II. Fill in the blanks)|5 VideosSTRUCTURE OF THE ATOM
CPC CAMBRIDGE PUBLICATION|Exercise UNIT TEST (II. Answer the following)|3 VideosMATTER IN OUR SURROUNDINGS
CPC CAMBRIDGE PUBLICATION|Exercise UNIT TEST (Answer the following)|4 Videos
Similar Questions
Explore conceptually related problems
CPC CAMBRIDGE PUBLICATION-STRUCTURE OF THE ATOM -EXERCISE
- Compare the properties of electrons, protons and neutrons.
Text Solution
|
- What is the limitation of Thomson's model of atom.
Text Solution
|
- What are the limitations of Rutherford's model?
Text Solution
|
- Summarize the Bohr's Model of an atom.
Text Solution
|
- Compare all the proposed model of an atom given in this chapter.
Text Solution
|
- Summarize the rule for writing of distribution of electrons in various...
Text Solution
|
- Define valency by taking examples of silicon and oxygen .
Text Solution
|
- Explain with examples (a) Atomic number Mass number Isotopes
Text Solution
|
- Na^(+) has completely filled K and L shells explain ?
Text Solution
|
- If Bromine atom is available in the form of say, two isotopes .(35)^(7...
Text Solution
|
- The average atomic mass of a sample of an element X is 16.2 u. What ar...
Text Solution
|
- If Z = 3, what would be the valency of the element ? Also, name the el...
Text Solution
|
- Composition of the nuclei of two atomic species X and Y are given as u...
Text Solution
|
- For the following statements, write True (T) or False (F). If the stat...
Text Solution
|
- Rutherford's alpha - particle scattering experiment was responsible fo...
Text Solution
|
- Isotopes of an element have
Text Solution
|
- Number of valence electrons in ion are
Text Solution
|
- Which one of the following is a correct electronic configuration of so...
Text Solution
|
- Complete the following table
Text Solution
|