Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CPC CAMBRIDGE PUBLICATION-ACIDS, BASES AND SALTS-UNIT TEST (Answer the following questions :)
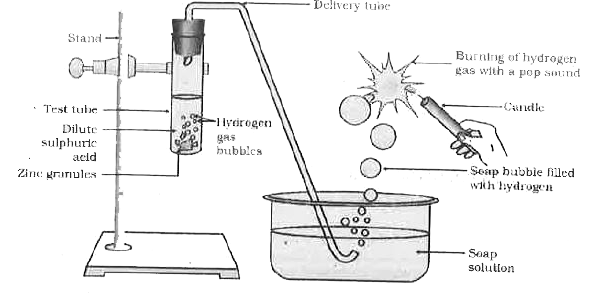
- Which gas is usually liberated when an acid reacts with a metal ? Illu...
Text Solution
|
- Why does dry HCl gas not change the colour of the dry litmus.
Text Solution
|
- Write word equations and then balance equations for the reaction takin...
Text Solution
|
- Plaster of Paris should be stored in a moisture-proof container. Expla...
Text Solution
|
- What is bleaching powder? How is it prepared? List two uses of bleachi...
Text Solution
|
- What will happen if a solution of sodium hydro carbonate is heated ? G...
Text Solution
|