Text Solution
Verified by Experts
Topper's Solved these Questions
ACIDS, BASES AND SALTS
CPC CAMBRIDGE PUBLICATION|Exercise UNIT TEST (Fill in the blanks)|3 VideosACIDS, BASES AND SALTS
CPC CAMBRIDGE PUBLICATION|Exercise UNIT TEST (Answer the following )|2 VideosACIDS, BASES AND SALTS
CPC CAMBRIDGE PUBLICATION|Exercise ADDITIONAL QUESTIONS|1 VideosCARBON AND ITS COMPOUNDS
CPC CAMBRIDGE PUBLICATION|Exercise UNIT TEST(Answer the following)|8 Videos
Similar Questions
Explore conceptually related problems
CPC CAMBRIDGE PUBLICATION-ACIDS, BASES AND SALTS-ADDITIONAL QUESTIONS (Answer the following questions :)
- What are amphoteric substances? Illustrates with an example.
Text Solution
|
- On the basis of origin, how acids are classified?
Text Solution
|
- Why vinegar is used in pickling?
Text Solution
|
- Define dilution.
Text Solution
|
- (a) Write the common name of CaOCl2 How it is prepared? Write the chem...
Text Solution
|
- W'hat are organic acids? Give two examples.
Text Solution
|
- How does a strong acid differ from a concentrated acid?
Text Solution
|
- Why should curd and sour substances not be kept in brass and copper ve...
Text Solution
|
- Why should acids be handled with care?
Text Solution
|
- While diluting an acid, why is it recommended that the acid should be ...
Text Solution
|
- 15 ml of water and 10 ml of Sulphuric acid are mixed in a beaker. (...
Text Solution
|
- A white coloured powder is used by doctors for supporting fractured bo...
Text Solution
|
- (i) Write the name given to bases that are highly soluble in water. Gi...
Text Solution
|
- (i) Give the constituents of baking powder. (ii) Why cake or bread s...
Text Solution
|
- How will you test for the gas which is liberated when hydrochloric aci...
Text Solution
|
- Sodium hydrogen carbonate is a basic salt. Justify the statement how i...
Text Solution
|
- Mention the colour of FeSO(4). 7H(2)O crystals . How does this colour ...
Text Solution
|
- What happens when chlorine is passed over slacked lime at 313k? Write ...
Text Solution
|
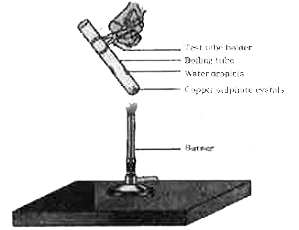
- What is meant by water of Crystallisation ? How do you show that coppe...
Text Solution
|
- Why does 1 m HCI solution have a higher concentration of H^(+) ions th...
Text Solution
|