Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
CPC CAMBRIDGE PUBLICATION|Exercise EXERCISE|20 VideosMETALS AND NON-METALS
CPC CAMBRIDGE PUBLICATION|Exercise ADDITIONAL QUESTIONS ( CHOOSE THE CORRECT ANSWER )|10 VideosMARCH - 2019 QUESTION PAPER - 10
CPC CAMBRIDGE PUBLICATION|Exercise QUESTIONS|40 VideosMOST LIKELY QUESTION PAPER - 9
CPC CAMBRIDGE PUBLICATION|Exercise QUESTIONS|37 Videos
Similar Questions
Explore conceptually related problems
CPC CAMBRIDGE PUBLICATION-METALS AND NON-METALS-UNIT TEST
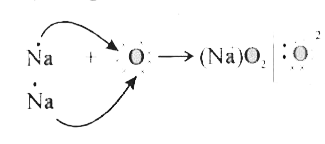
- (i) Write the electron dot structure for sodium, oxygen and magnesium....
Text Solution
|
- What are noble metals?
Text Solution
|
- Give reasons for the following : (i) School bells are made up of met...
Text Solution
|
- Give examples of the least reactive metals.
Text Solution
|
- Define the following terms. (i) Mineral (ii) ore (iii) Gangue
Text Solution
|
- State two ways to prevent the rusting of iron.
Text Solution
|
- Differentiate between metal and non-metal on the basis of chemical pro...
Text Solution
|
- Give reasons a. Platinum, gold and silver are used to make jewellery...
Text Solution
|
- You are provided with magnesium ribbon and sulphur powder. Explain wit...
Text Solution
|