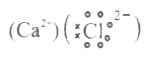Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
CPC CAMBRIDGE PUBLICATION|Exercise UNIT TEST|8 VideosMETALS AND NON-METALS
CPC CAMBRIDGE PUBLICATION|Exercise ADDITIONAL QUESTIONS ( CHOOSE THE CORRECT ANSWER )|10 VideosMARCH - 2019 QUESTION PAPER - 10
CPC CAMBRIDGE PUBLICATION|Exercise QUESTIONS|40 VideosMOST LIKELY QUESTION PAPER - 9
CPC CAMBRIDGE PUBLICATION|Exercise QUESTIONS|37 Videos
Similar Questions
Explore conceptually related problems
CPC CAMBRIDGE PUBLICATION-METALS AND NON-METALS-ADDITIONAL QUESTIONS ( Answer the following)
- a. Write electron dot diagram for chlorine (At No. 17) and Calcium (At...
Text Solution
|
- Identify the nature of above compound and explain three physical prope...
Text Solution
|
- Give reasons for the following : (i) School bells are made up of met...
Text Solution
|
- Give reasons for the following : (i) School bells are made up of met...
Text Solution
|
- What asre amphoteric oxides? Choose the amphoteric oxide from among th...
Text Solution
|
- Differentiate between roasting and calcination. Explain the two with...
Text Solution
|
- Name two metals than can be used to reduce metal oxides of metals.
Text Solution
|
- State reasons for the following: Electric wires are covered with rub...
Text Solution
|
- Which of the following metals cannot liberate hydrogen from dilute ...
Text Solution
|
- State reasons for the following: Sulphide ore of a metal is first co...
Text Solution
|
- Write any four physical properties of metals.
Text Solution
|
- Give examples of the least reactive metals.
Text Solution
|
- Sodium and chlorine are poisonous substances but sodium chloride is ed...
Text Solution
|
- Write balanced equations for the reactions of : (i) Aluminium when h...
Text Solution
|
- Write balanced chemical equation for the reaction of: a. Al when hea...
Text Solution
|
- Write balanced equations for the reactions of : (i) Aluminium when h...
Text Solution
|
- (i) Explain the formation of ionic compound CaO with electron dot stru...
Text Solution
|
- Name the constitutent metals of bronze.
Text Solution
|
- a. Give two methods to prevent the rusting of iron b. Name the ores ...
Text Solution
|
- You are provided with magnesium ribbon and sulphur powder. Explain wit...
Text Solution
|