Similar Questions
Explore conceptually related problems
Recommended Questions
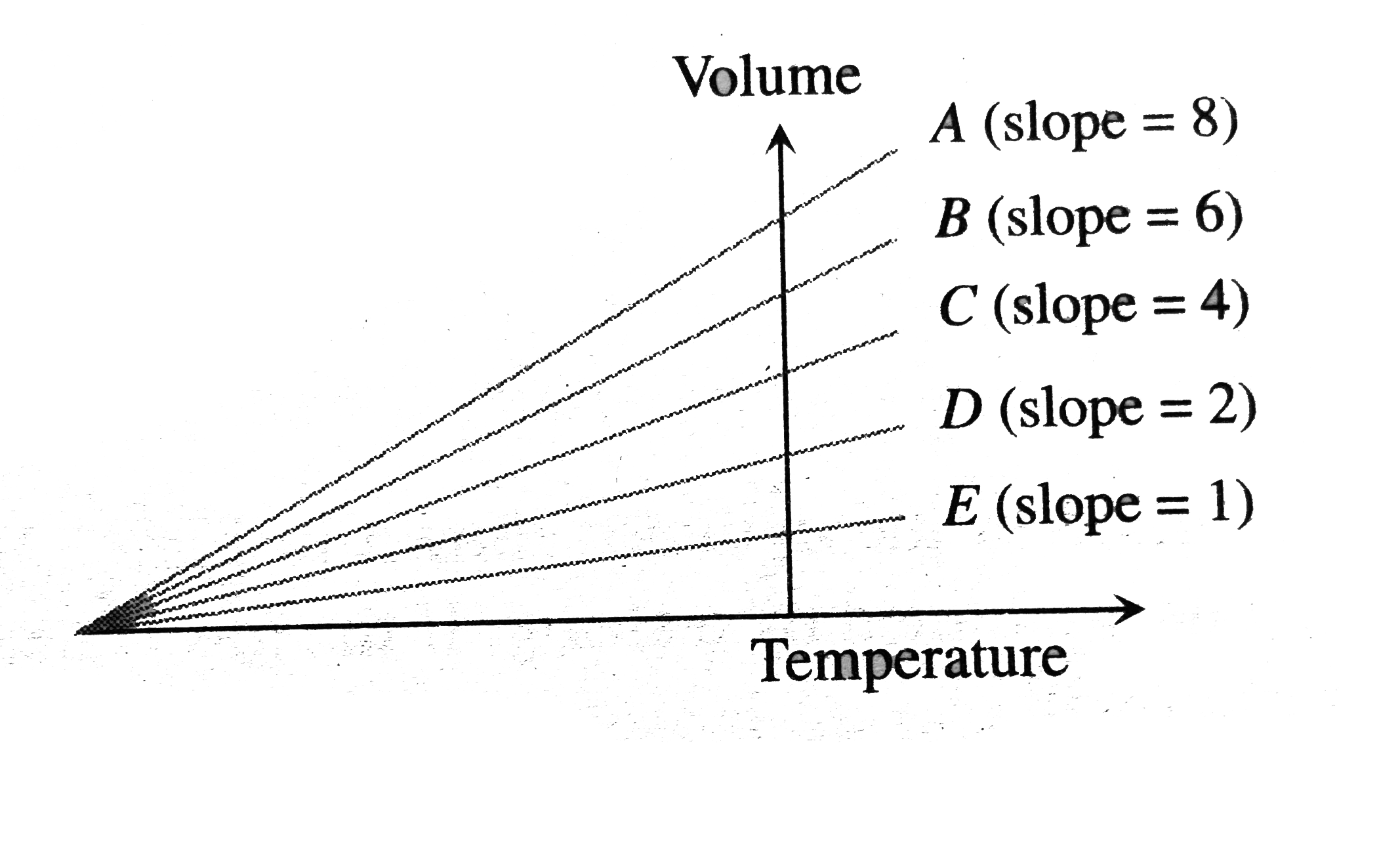
- The expansion of an ideal gas of mass m at a constant pressure P is gi...
Text Solution
|
- The expansion of an ideal gas of mass m at a constant pressure P is gi...
Text Solution
|
- STATEMENT-1 : Plot of P vs 1/V (volume) is a straight line for an idea...
Text Solution
|
- The expansion of an ideal gas of mass ma t a constant pressur p is giv...
Text Solution
|
- If P, V, M, T and R are symbols of pressure, volume, molecular weight,...
Text Solution
|
- यदि P, V, M, T तथा R क्रमशः दाब, आयतन मोलर द्रव्यमान, ताप तथा गैस स्थ...
Text Solution
|
- A plot of P vs T for a given mass of gas at constant volume is a strai...
Text Solution
|
- The plot of pressure (P) versus temperature (T) for an ideal gas will ...
Text Solution
|
- The expansion of an ideal gas of mass m at a constant pressure P is gi...
Text Solution
|