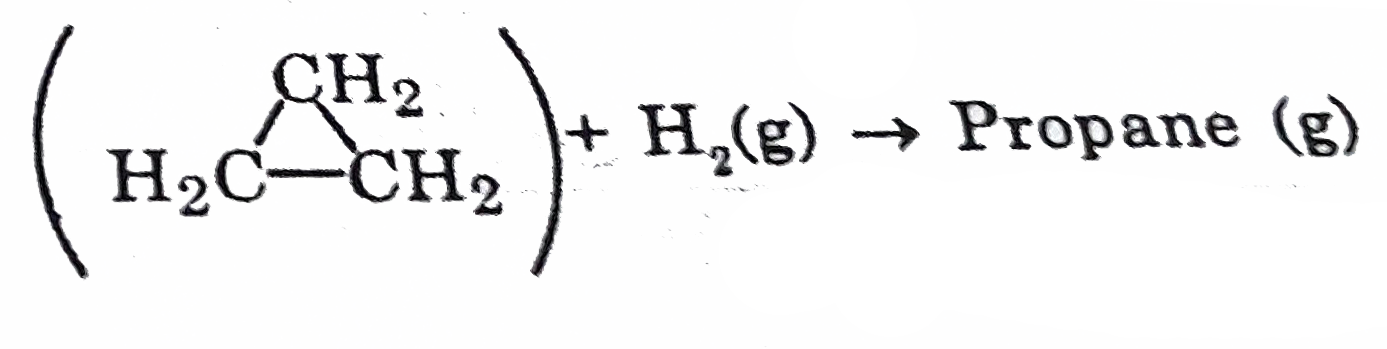Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate DeltaH for the reaction - Cyclopropane (g) Give...
Text Solution
|
- Using the bond enthalpy data given below, calculate the enthalpy of fo...
Text Solution
|
- Calculate DeltaH for the reaction - Cyclopropane (g) Given : {:("Bond"...
Text Solution
|
- Delta H for the reaction H-C-= N(g) + 2H(2)(g) rarr H - underset(H) un...
Text Solution
|
- Assertion.The bond enthalpy of C-H bondin CH(4) is nearly 416 k J mol^...
Text Solution
|
- The bond enthalpy of C - C, C = C, H - H and C - H bonds are 350,600,4...
Text Solution
|
- The bond energies of C-C,C=C, H-H and C-H bonds are 350 kJ mol^-1, 600...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- If the bond energy of C-H bond is +416.18 kJ*mol^(-1), then what will ...
Text Solution
|