Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-REDOX REACTION-Exercise -4
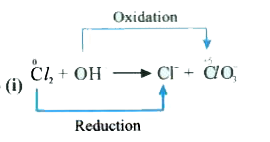
- In passing chlorine gas through a concentrated solution of alkali we g...
Text Solution
|
- Which of the following represents a redox reaction?
Text Solution
|
- In the reaction SO(2)+2H(2)Srarr3S+2H(2)O the substance oxidised i...
Text Solution
|
- In the reaction 3Cl(2)+6NaOHrarrNaClO(3)+5NaCl+3H(2)O the element ...
Text Solution
|
- The oxidation number of oxygen in OF(2) is
Text Solution
|
- An oxidation process involves
Text Solution
|
- Which of the following is the strongest reducing agent in aqueous medi...
Text Solution
|
- Which of the following is the strongest oxidising agent?
Text Solution
|
- The oxidation number of phosphorus in Ba(H(2)PO(2))(2) is:-
Text Solution
|
- Which of the following reactions do not involve oxidation reduction ? ...
Text Solution
|
- For the redox reaction MnO(4)^(ө)+C(2)O(4)^(2-)+H^(o+)rarrMn^(2+)+CO...
Text Solution
|
- The oxidation state of nitrogen is correctly given for
Text Solution
|
- The oxidation state of chromium in Cr(CO)(6) is
Text Solution
|
- Which of the following is not a redox reaction?
Text Solution
|
- In the chemical reaction, K(2)Cr(2)O(7)+xH(2)SO(4)+ySO(2)rarrK(2)SO(...
Text Solution
|
- One mole of N(2)H(4) loses ten moles of electrons of form a new compou...
Text Solution
|
- When copper is treated with a certain concentration of nitric acid, ni...
Text Solution
|
- In which of the following pairs is there the greatest difference in th...
Text Solution
|
- In the reaction 3Br(2) + 6CO(3)^(2-) + 3H(2)O to 5Br^(-) + 2BrO(3)^(...
Text Solution
|
- In the reaction 2FeCl(3)+H(2)Srarr2FeCl(2)+2HCl+S
Text Solution
|
- The oxidation number of cobalt in K[Co(CO)(4)] is:
Text Solution
|