A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-REDOX REACTION-Exercise -3
- Oxidation state of oxygen in F2O is
Text Solution
|
- When sulphur dioxide is passed in an acidified K2Cr2O7 solution,the ox...
Text Solution
|
- Oxidation number if iodine in IO(3)^(-), IO(4)^(-),KI and I(2) respect...
Text Solution
|
- Oxidation state of P in H(4)P(2)O(5), H(4)P(2)O(6), H(4)P(2)O(7) are r...
Text Solution
|
- Which of the following have been arranged in the decreasing order of o...
Text Solution
|
- The highest oxidation state of Mn is shown by
Text Solution
|
- Chlorine is in +3 oxidation state in
Text Solution
|
- How many moles of iodine are liberated when one mol of potassium dichr...
Text Solution
|
- For the redox reaction, MnO(4)^(-) + C(2)O(4)^(2-) + H^(+) rarr Mn^(...
Text Solution
|
- An oxidation process involves
Text Solution
|
- Which of the following is the most powerful oxidising agent?
Text Solution
|
- Which of the following reaction involves oxidation reduction?
Text Solution
|
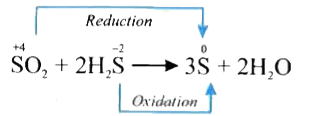
- In the reaction SO(2)+2H(2)Srarr3S+2H(2)O the substance oxidised i...
Text Solution
|
- The value of n in : MnO(4)^(-)+8H^(+)+n erarr Mn^(2+)+4H(2)O is
Text Solution
|
- Which can act as an oxidising as well as a reducing agent ?
Text Solution
|
- Which change requires an oxidising agent?
Text Solution
|
- In the reaction , 2H(2)O(2) to 2H(2)O + O(2) , oxygen is
Text Solution
|
- In chromite ore, the oxidation number of iron and chromium are respect...
Text Solution
|
- In which of the following compounds, nitrogen exhibits highest oxidati...
Text Solution
|
- Which of the following species can function both as oxidizing as well ...
Text Solution
|