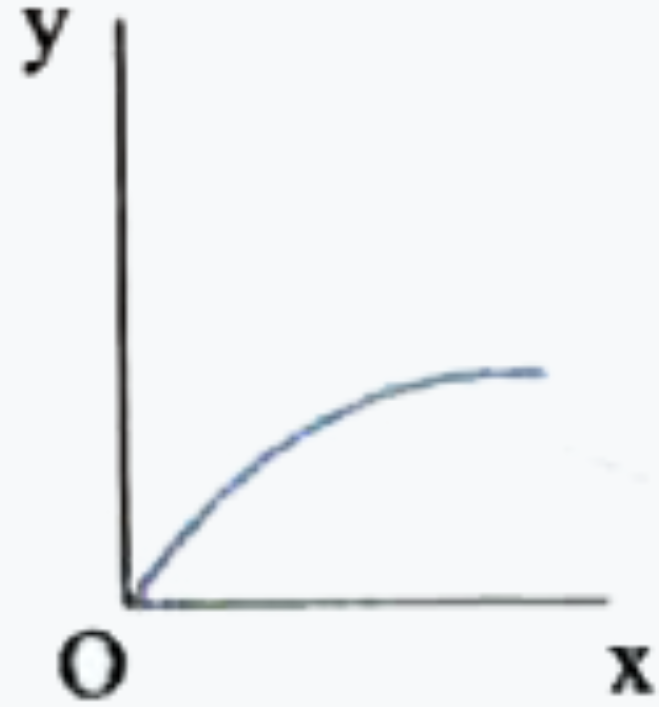
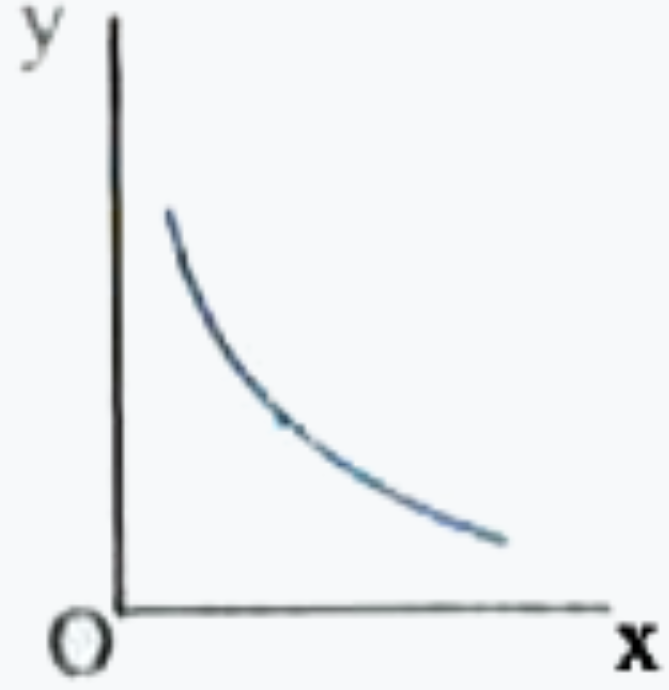
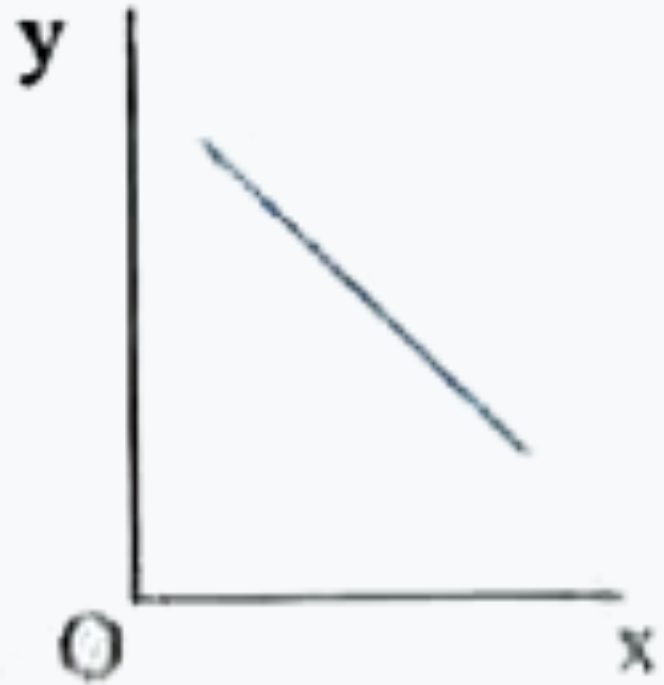
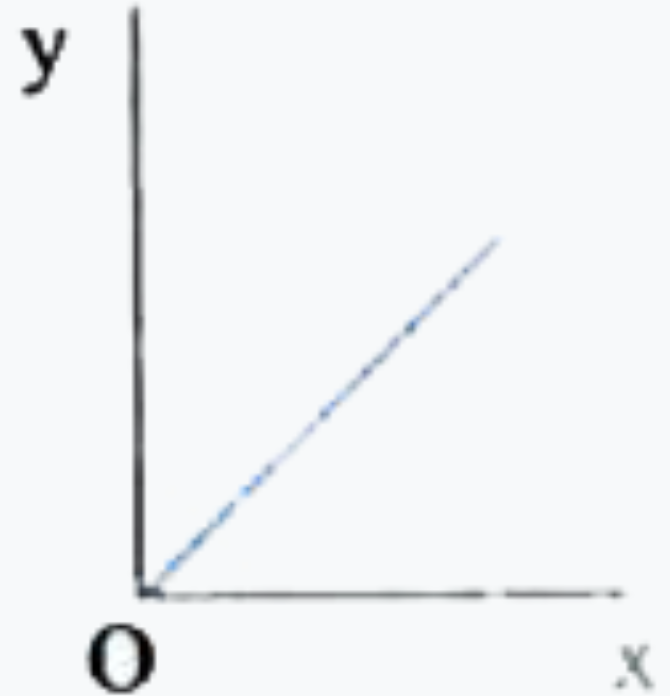
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS & COLLIGATIVE PROPERTIES
NARAYNA|Exercise EXERCISE : 1 (H.W)|29 VideosSOLUTIONS & COLLIGATIVE PROPERTIES
NARAYNA|Exercise EXERCISE : 2 (C.W)|65 VideosSOLUTIONS & COLLIGATIVE PROPERTIES
NARAYNA|Exercise C.U.Q.|40 VideosSOLID STATES
NARAYNA|Exercise EXERCISE-4|18 VideosSURFACE CHEMISTRY
NARAYNA|Exercise EXERCISE - 4|22 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-SOLUTIONS & COLLIGATIVE PROPERTIES -EXERCISE : 1 (C.W)
- The density of 2M solution of acetic acid (Mol. Wt. 60) is 1.02 g ml^(...
Text Solution
|
- Which of the following is correct ?
Text Solution
|
- Which of the following has no unit?
Text Solution
|
- What is the mole fraction of glucose in 10% w/W glucose solution ?
Text Solution
|
- Calculate the weight of carbon dioxide dissolved in 1 L bottle of carb...
Text Solution
|
- The boiling point of C(6)H(6) , CH(3)OH , C(6)H(5)NH(2) "and"C(6)H(5)N...
Text Solution
|
- A solution is obtained by dissolving 12gof urea(mol.wt.60)in a litre o...
Text Solution
|
- Lowering of vapour pressures of equimolar solution of glucose, sodium ...
Text Solution
|
- Which of the following salt has the same value of Van’t Hoff factor ‘i...
Text Solution
|
- Which one of the following represents the graph between log P on Y - a...
Text Solution
|
- If an ideal solution is made by mixing 2 moles of benzene (p^(@)=266mm...
Text Solution
|
- The molecular mass of NaCI as determined by osmotic pressure measureme...
Text Solution
|
- Which among the following will show maximum osmotic pressure ?
Text Solution
|
- An aqueous solution of 2 per cent (wt.//wt) non-volatile solute exerts...
Text Solution
|
- Pure water boils at 99.725^(@)C at Shimla. If K(b) for water is 0.51 K...
Text Solution
|
- Two elements A and B form compounds having molecular formula AB(2) and...
Text Solution
|
- The amount of ice that will separate out on cooling a solution contain...
Text Solution
|
- The osmotic pressure of solution at 0^(@)C is 4 atm what will be its o...
Text Solution
|
- The molar mass of a solute X in g mol^(-1), if its 1% solution is isot...
Text Solution
|
- Molal elevation constant "K"(b) for water is 0.52K//m. 0.1 molal solut...
Text Solution
|