A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMICAL KINETICS -EXERCISE -4
- Consider the Arrhenius equation given below and mark the correct op...
Text Solution
|
- Which of the following statement is not correct about order of a ...
Text Solution
|
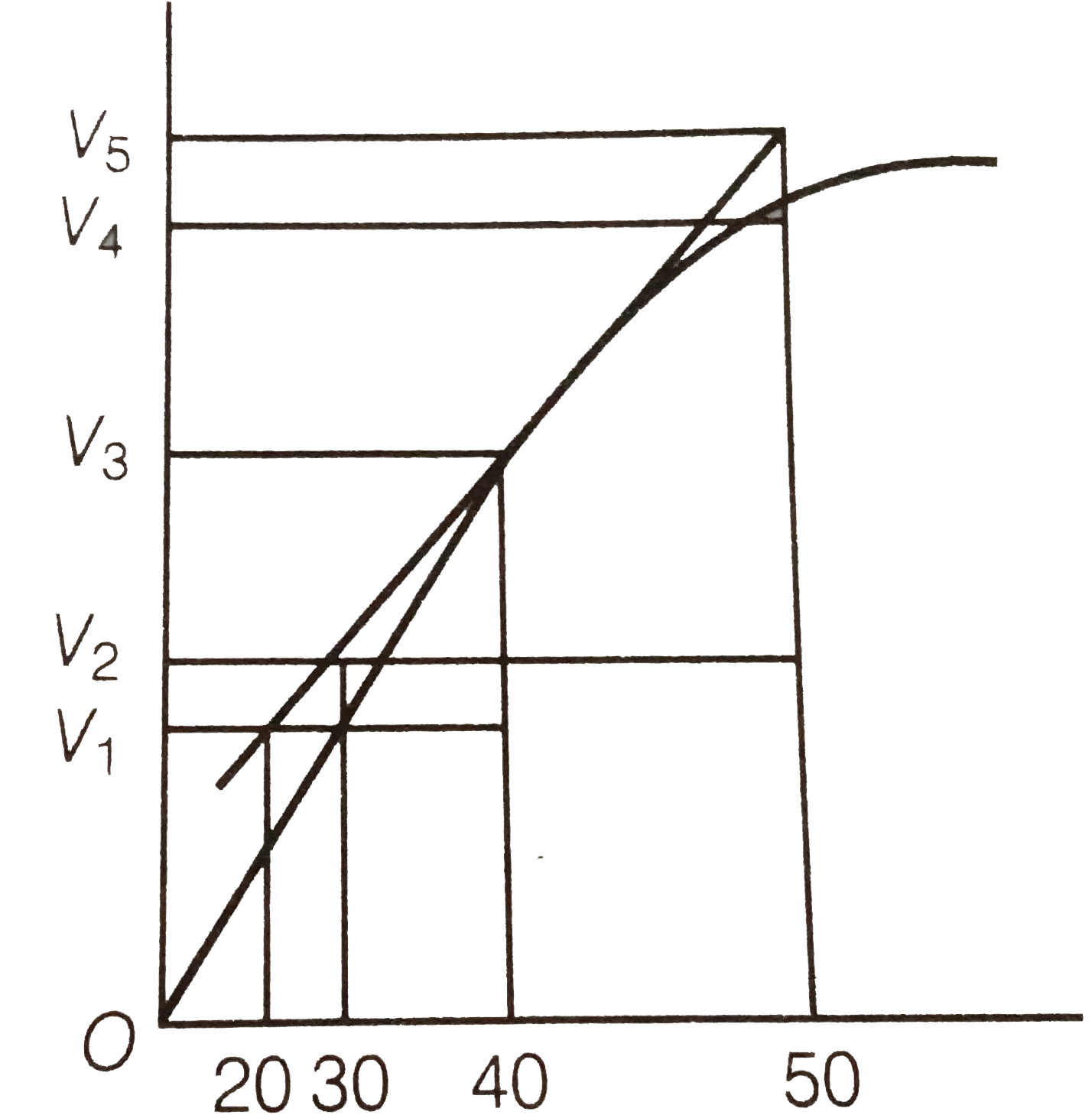
- Consider the graph given in figure . Which of the following optio...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following expression is correct for the rate of reac...
Text Solution
|
- Rate law for the reaction, A + 2B to C is found to be Rate = k [A] [...
Text Solution
|
- Which of the following statements is incorrect about the collison theo...
Text Solution
|
- A first order reaction is 50% completed in 1.26 xx 10^(14)s. How much ...
Text Solution
|
- Which of the following statements is not correct for the catalyst?
Text Solution
|
- The value of rate constant of a pseudo first order reaction
Text Solution
|
- Consider the reaction A to B. The concentration of both the reactan...
Text Solution
|
- The time for half-life period of a certain reaction, A rarr products i...
Text Solution
|
- The rate of a chemical reaction doubles for every 10^(@)C rise of temp...
Text Solution
|
- A reactant (A) forms two products A overset(K(1)) to B, Activation en...
Text Solution
|
- For a reaction 1//2A to 2B, rate of disappearance of A is related to t...
Text Solution
|
- For the reaction A + 2B + CtoD+2E the rate equation is rate =K[A][B]^(...
Text Solution
|
- The acid hydrolysis of ester is: (i) first order reaction (ii) bimolec...
Text Solution
|
- Which of the following statements are correct: (i) law of mass action ...
Text Solution
|
- Consider the following reactions at 300 K. A rarr B (uncatalysed rea...
Text Solution
|
- A substan ce undergoes first order decomposition. The decomposition fo...
Text Solution
|