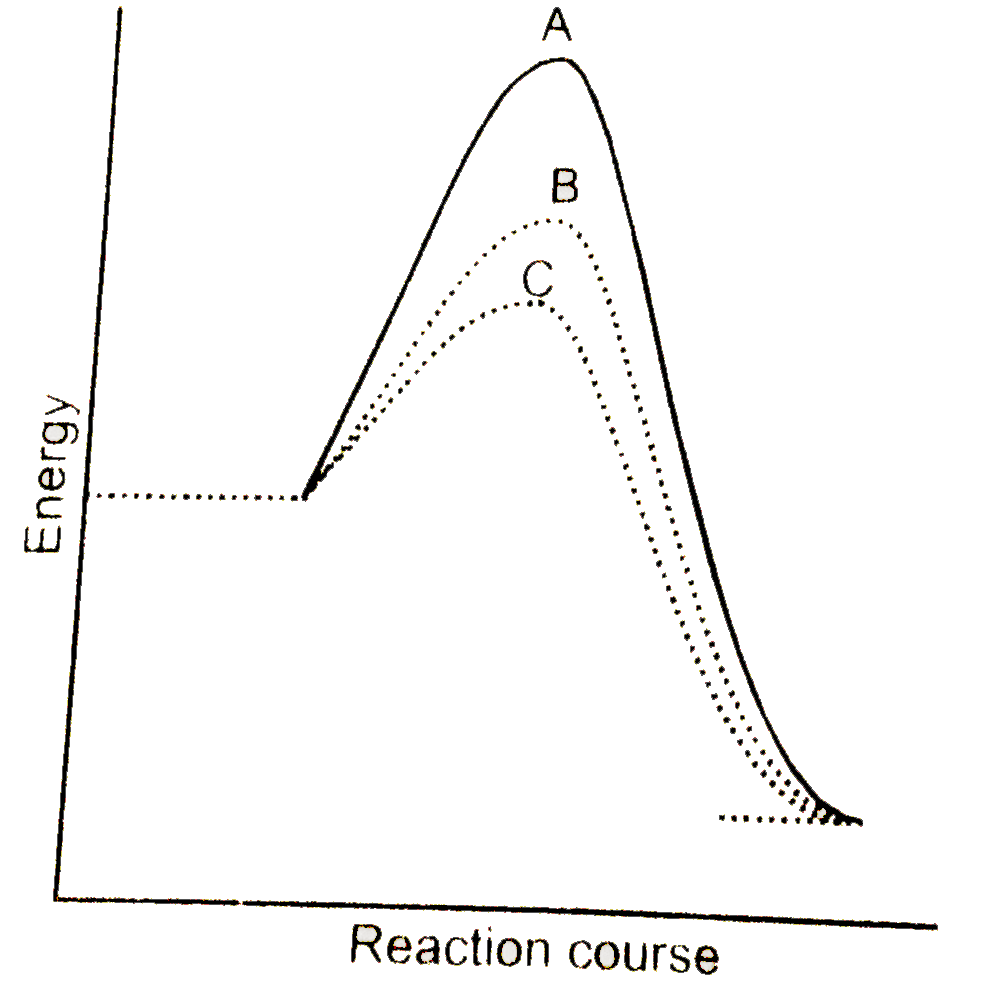
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-SURFACE CHEMISTRY -EXERCISE -2 (C.W. ) ADSORPTION
- Chromatography is a technique based on
Text Solution
|
- The volume of gases H(2), CH(4), CO(2) and NH(3) adsorbed by 1 g of ch...
Text Solution
|
- Which of the following processes of metallurgy involves adsorption ?
Text Solution
|
- According to langmuir adsorption isotherm amount of gas adsorbed at ve...
Text Solution
|
- Which of the following relations is /are correct? (i) x//m= constant...
Text Solution
|
- A catalyst :
Text Solution
|
- In a reversible reaction a catalyst :-
Text Solution
|
- Which of the following is not an example of homogeneous catalysis?
Text Solution
|
- The decomposition of hydrogen peroxide can be slowed by the addition o...
Text Solution
|
- Freundlich adsorption isotherm is given by the expression (x)/(m) = kP...
Text Solution
|
- In which of the following process, a catalyst is not used ?
Text Solution
|
- Adsorption is a surface phenomenon and it differs feom adsorption whic...
Text Solution
|
- Organic catalysts differ from inorganic catalysts
Text Solution
|
- Which one of the following is not a homogeneous catalytic reaction ?
Text Solution
|
- Decomposition of urea into NH(3) and CO(2) is followed by the action o...
Text Solution
|
- Which of the following enzyme is used in the conversion of proteins to...
Text Solution
|
- Zeolites are microporous catalyst. General formula of Zeolite may be g...
Text Solution
|
- CH(3)COCI+H(2)overset(Pd)(rarr)CH(3)CH(2)OH overset(Pd//BaSO(4))und...
Text Solution
|
- An example of auto - catalytic reaction is
Text Solution
|
- A homogenous catalytic reaction takes place through the three alternat...
Text Solution
|