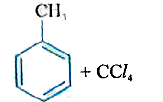
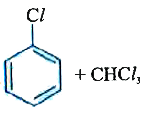
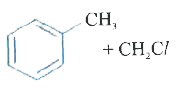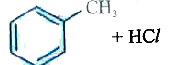A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANE AND HALOARENES
NARAYNA|Exercise EXERCISE - II (H.W)|28 VideosHALOALKANE AND HALOARENES
NARAYNA|Exercise EXERCISE - 3|37 VideosHALOALKANE AND HALOARENES
NARAYNA|Exercise EXERCISE - I (H.W)|33 VideosF-BLOCK ELEMENTS
NARAYNA|Exercise CHECK YOUR GRASP|2 VideosHALOGEN COMPOUNDS
NARAYNA|Exercise Passage 13|1 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-HALOALKANE AND HALOARENES-EXERCISE - II (C.W)
- What is the major product of the following reaction ? CH(3)C-=C-CH(2)...
Text Solution
|
- The reaction C2H5OH+SOCl2overset("Pyridine")rarrC2H5Cl+SO2+HCl is know...
Text Solution
|
- When CH3CH2CHCl2 is treated with NaNH2, the product formed is
Text Solution
|
- CH(3)CH(2)Cloverset(NaCN)rarrXoverset(Ni//H(2))rarrYunderset("anhydrid...
Text Solution
|
- Benzene reacts with chlorine to form benzene hexachloride in presence ...
Text Solution
|
- Chloroform when treated with aniline and alcoholic KOH forms -
Text Solution
|
- The reaction C2H5ONa+BrC2H5rarrC2H5-O-C2H5+NaBr is called
Text Solution
|
- Identify Z in the following series: C(2) H(5) I underset("KOH")over...
Text Solution
|
- What is X in the following reaction ? C2H5Cl +X rarrC2H5OH + KCl
Text Solution
|
- 1 - Chloroprapane and 2 - chloropropane are
Text Solution
|
- The compound , pyrene, used in fire extinguishers is
Text Solution
|
- On sulphonation of C6H5 Cl
Text Solution
|
- Which is the carbylamine reaction ?
Text Solution
|
- The product formed in the following reaction is CH3CH(CH3)CH=CH2+HBrra...
Text Solution
|
- Total number of acyclic isomers including stereoisomers of AlCl3 find ...
Text Solution
|
- During Friedel craft reaction of Benzene with CH3Cl in presence of AlC...
Text Solution
|
- Chlorobenzene reacts with Mg in dry ether to give a compound A, which ...
Text Solution
|
Text Solution
|
- What is C ?
Text Solution
|
- The correct orders of reactivity tow ards SN1 reaction is
Text Solution
|