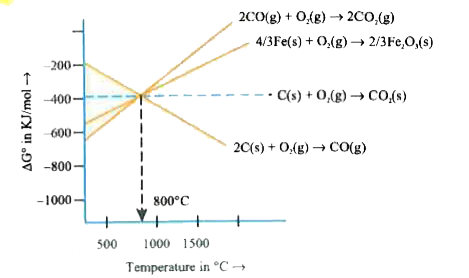
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
METALLURGY
NARAYNA|Exercise EXERCISE - 2 (REFINING OF METALS AND USES)|6 VideosMETALLURGY
NARAYNA|Exercise EXERCISE - II (HOME WORK)|9 VideosMETALLURGY
NARAYNA|Exercise EXERCISE - 2 (CONCENTRATION OF ORES)|4 VideosHALOGEN COMPOUNDS
NARAYNA|Exercise Passage 13|1 VideosMETTALURGY
NARAYNA|Exercise Statement Typer Question|2 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-METALLURGY-EXERCISE - 2 (EXTRACTION OF METALS)
- Which of the following processes involves smelting ?
Text Solution
|
- Identify X and Y in the following reactions . PbS underset("in air")...
Text Solution
|
- Heating mixture of Cu(2)Oand Cu(2)S will give
Text Solution
|
- Extraction of zinc from zinc blende is achieved by:
Text Solution
|
- Which of the following is correctly matched?
Text Solution
|
- How is cas iron different from pig iron ?
Text Solution
|
- Carbon reduction process is used for the extraction of
Text Solution
|
- When ZnS and PbS minearls are present together, then NaCN is added to ...
Text Solution
|
- Consider the following reaction at 1000^(@)C (A) Zn (s) + (1)/(2) O...
Text Solution
|
- The smelting of iron in a blast furnace involves, which of the followi...
Text Solution
|
- The maximum temperature 1550^(@)C is obtained in the region of the bl...
Text Solution
|
- In the commercial electrochemical process for aluminium extraction, th...
Text Solution
|
- What is the effect of adding a catalyst on (a) Activation energy (Ea...
Text Solution
|
- निम्नलिखित में से कोनसा कथन सही है----
Text Solution
|
- Correct statements form the graph I) Above 1073K, DeltaG^(0) for...
Text Solution
|