A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CO-ORDINATE COMPOUNDS -Exercise-3
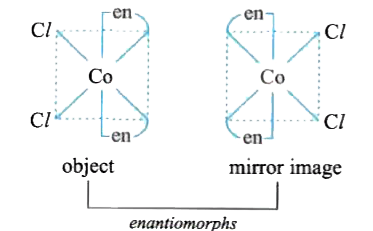
- Which of the following will give a pair of enontiomorphs ? en = NH(2...
Text Solution
|
- Which of the following is organometallic compound
Text Solution
|
- Which of the following complexes exhibits the highest paramagnetic beh...
Text Solution
|
- In which of the following coordination entities the magnitude of Delta...
Text Solution
|
- Assertion: [CoF(6)]^(3-) is a paramagnetic. Reason: Co^(3+)" has 3d"...
Text Solution
|
- Which of the following does not show optical isomerism ?
Text Solution
|
- Which of the following complex ions is expected to absorb visible ligh...
Text Solution
|
- Which of the following coordination entities should be expected to abs...
Text Solution
|
- Assertion : CO and CN^(-) are referred as p acid L ligands. Reason :...
Text Solution
|
- Assertion : If beta(4)" for "[Cu(NH3)(4)]^(2+)" is "2.1 xx 10^(13) its...
Text Solution
|
- Assertion: K(4)[Fe(CN(6)] is diamagnetic and [Fe(H(2)O)(6)]Cl(3) is pa...
Text Solution
|
- What is (are) number (s) of unpaired electron(s) in the square planar ...
Text Solution
|
- IUPAC name of [Fe(CN)(6)]^(3-) ion is
Text Solution
|
- Crystal field stabilization energy for high spin d^4 octahedral comple...
Text Solution
|
- The existence of two different coloured comlexes with the composition ...
Text Solution
|
- Which of the following complex ion is not expected to absorb visible l...
Text Solution
|
- AgCl dissolved in excess of NH(3), KCN and Na(2)S(2)O(3) solutions the...
Text Solution
|
- The most stable complex among the following
Text Solution
|
- Choose the correct statement
Text Solution
|
- In the complex ion [CoNH(3))(6)]^(3+) , the NH(3) molecules are li...
Text Solution
|