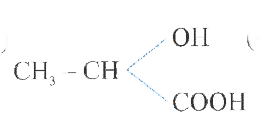
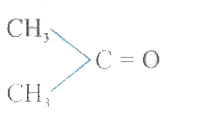A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise EXERCISE -I (H.W.)|15 VideosALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise EXERCISE -II (C.W.)|25 VideosALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise C.U.Q|60 VideosALCOHOLS, ETHERS, PHENOL
NARAYNA|Exercise Assertion Reaosn|10 VideosAMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Subjective type Q.|13 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-ALDEHYDES, KETONES & CARBOXYLIC ACIDS-EXERCISE -I (C.W.)
- Which of the following is correct for carbonyl compounds ?
Text Solution
|
- Aldol condensation is given by :
Text Solution
|
- Formaldehyde can be distiguished from acetaldehyde by the use of:
Text Solution
|
- Acetaldehyde on treatment with HCN forms a cyanohydrin. What will be t...
Text Solution
|
- Benaldehyde and acetaldehyde can be distinguished by
Text Solution
|
- Which of the following types of carbonyl groups will produce an oxime ...
Text Solution
|
- Which is used as a preservative for biological specimens ?
Text Solution
|
- The reaction , CH(3)CHO + H(2) N - NH(2) rarr CH(3) = N.NH(2) is :
Text Solution
|
- Which reagent can convert gtCo group to gtC(C(6)H(5)) OH?
Text Solution
|
- Benzene on ozonolysis followed by hydrolysis with water in presence of...
Text Solution
|
- The reaction C(6) H(5) CHO + CH(3) CHO overset("dil.NaOH")(rarr) C(...
Text Solution
|
- What of the following is expected to be most highly ionised in water ?
Text Solution
|
- Reactivity of acids in esterification follows the order:
Text Solution
|
- An organic halide was treated with KCN and the product was boiled with...
Text Solution
|
- The correct order of decreasing boiling points of CH(3)CONH(2) (A), CH...
Text Solution
|