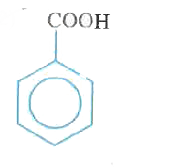
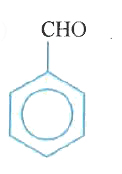
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise EXERCISE -4|30 VideosALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise CHECK YOUR GRASP|6 VideosALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise EXERCISE -II (H.W.)|25 VideosALCOHOLS, ETHERS, PHENOL
NARAYNA|Exercise Assertion Reaosn|10 VideosAMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Subjective type Q.|13 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-ALDEHYDES, KETONES & CARBOXYLIC ACIDS-EXERCISE -III
- The reaction CH(3) CH = CH(2) underset(H^(+))overset(CO + H(2)O)(rar...
Text Solution
|
- By oxidation with V(2) O(5), which one of the following gives phthalic...
Text Solution
|
- underset((ii) H(2)O)overset((i) CO(2))(rarr) In the reaction, product...
Text Solution
|
- Which of the following acids has the smallest dissociation constant
Text Solution
|
- CH(3)CH(2)COOH overset("Red P/HI")rarr is overset("alc. KOH")rarr "Pro...
Text Solution
|
- Acetophenone is prepared from :
Text Solution
|
- Glycerol reacts with potassium bisulphate to produce
Text Solution
|
- Identify the reactant X and the product Y CH(3) - CO - CH(3) + X rar...
Text Solution
|
- When m-chlorobenzaldehyde is treated with 50% KOH solution, the produc...
Text Solution
|
- A and B in the following reaction are R - C - R' overset("HCN/KCN"...
Text Solution
|
- Which gives lactic acid on hydrolysis after reacting with HCN in KCN ...
Text Solution
|
- A underset(800^(@)C )overset(Delta)(rarr) CH(2) = C = O , Reactant ' A...
Text Solution
|
- Laboratory method for the preparation of acetyl chloride is :
Text Solution
|
- 2CH(3) "COOH" underset(300^(@) C )overset(MnO)(rarr) A, product 'A ' i...
Text Solution
|
- Which of the aldehyde is most reactive ?
Text Solution
|
- CH(3) - CH(2) - C equiv CH underset(H(2)SO(4))overset("HgSO"(4))(rarr)...
Text Solution
|
- R - CH = CH(2) + CO + H(2) underset("High pressure" ) overset("High te...
Text Solution
|
- The regent used in Gattermann -Koch aldehyde synthesis is
Text Solution
|
- What is the main reason for the fact that carboxylic acids can undergo...
Text Solution
|
- On reductive ozonolysis yields
Text Solution
|