Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion : F bond angle p is equal to the bond angle Q but not preci...
Text Solution
|
- Assertion : F bond angle p is equal to the bond angle Q but not preci...
Text Solution
|
- Assertion : All F - S - F angle in SF(4) are greater than 90^(@) but ...
Text Solution
|
- Why is the repulsion between two lone pairs of electrons more than tha...
Text Solution
|
- Assertion : bond angle is less than the normal tetrahedral bond angle...
Text Solution
|
- कथन : NH(3) में प्रेक्षित आबन्ध कोण 109^(@)28' से कम होता है । ...
Text Solution
|
- The space model which is obtained by joining the points representing v...
Text Solution
|
- Assertion. All F-S-F bond angles in SF(4) are greater than 90^(@) but ...
Text Solution
|
- The approximate shape of a molecule can often be predicted by using wh...
Text Solution
|
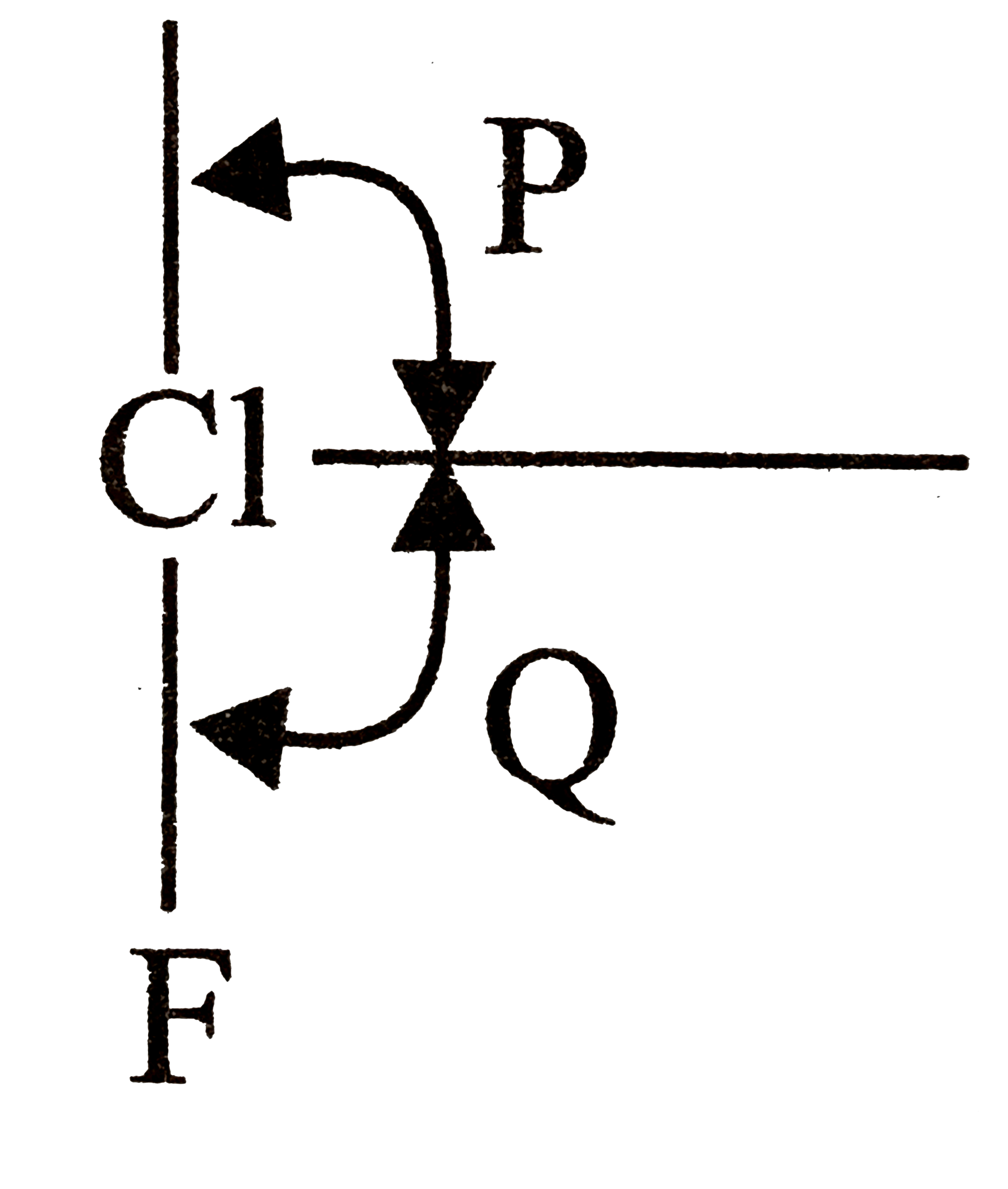 F bond angle p is equal to the bond angle Q but not precisely equal to `90^(@)`.
F bond angle p is equal to the bond angle Q but not precisely equal to `90^(@)`.