Similar Questions
Explore conceptually related problems
Recommended Questions
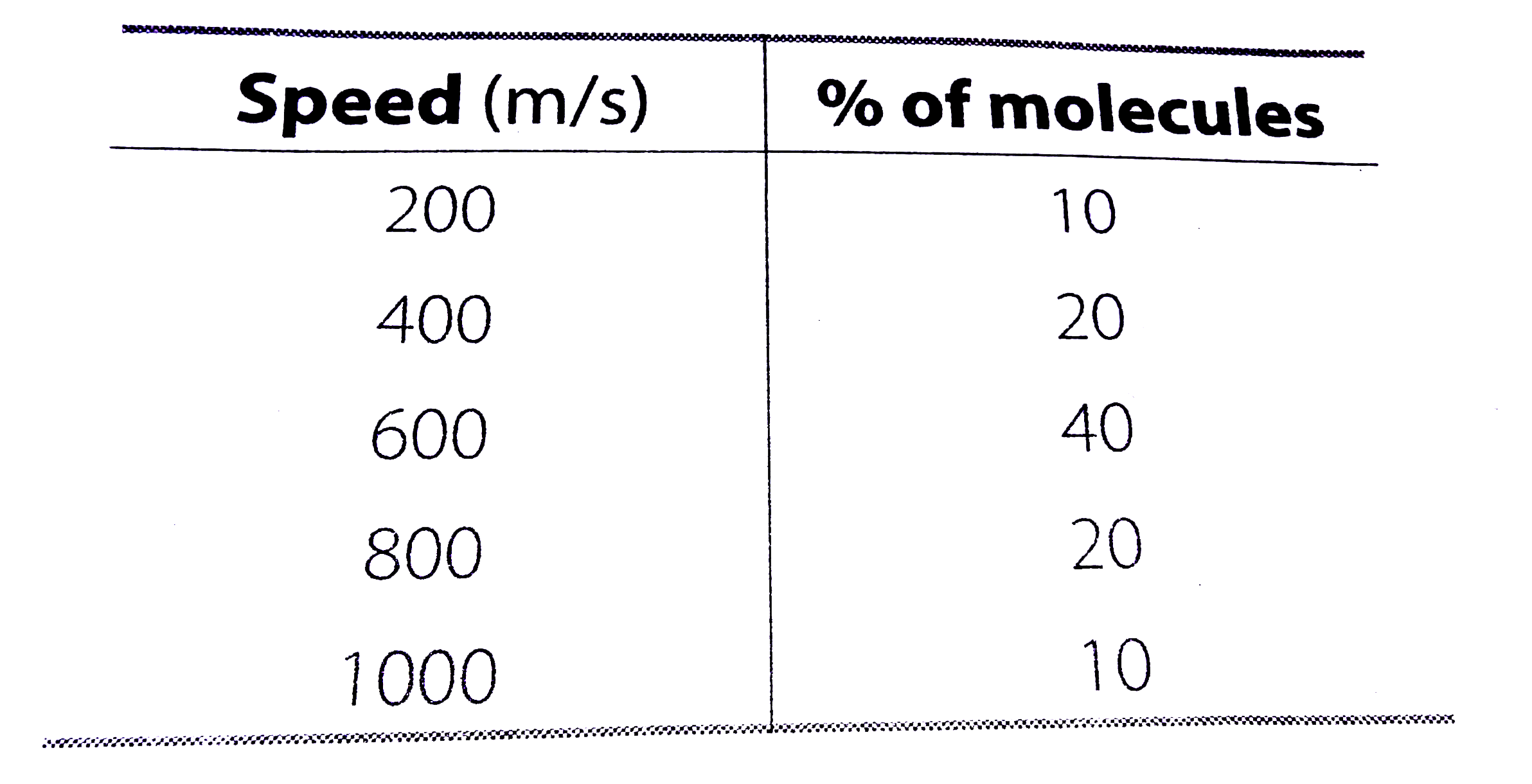
- Consider an ideal gas with following distribution of spedds. (a) C...
Text Solution
|
- Considering all type of D.O.F for HCl molecule of mass m having V(rms)...
Text Solution
|
- The rms speed of molecules of an ideal gas is v(rms) . Obtain the expr...
Text Solution
|
- Speed of sound wave in a gas V(1) and rms speed of molecules of the ga...
Text Solution
|
- Choose the correct relation between the rms speed (V(rms)) of the gas ...
Text Solution
|
- Consider an ideal gas with following distribution of spedds. (a) Calcu...
Text Solution
|
- If v(rms) is the rms speed of molecules in a gas and v is the speed of...
Text Solution
|
- ताप T पर अणुभार M की आदर्श गैस के अणुओं का वर्ग –माध्य-मूल चाल (v("rm...
Text Solution
|
- यदि गैस अणु का द्रव्यमान m हो तब ताप T पर गैसा के अणुओं की वर्ग-माध्य-...
Text Solution
|