Similar Questions
Explore conceptually related problems
Recommended Questions
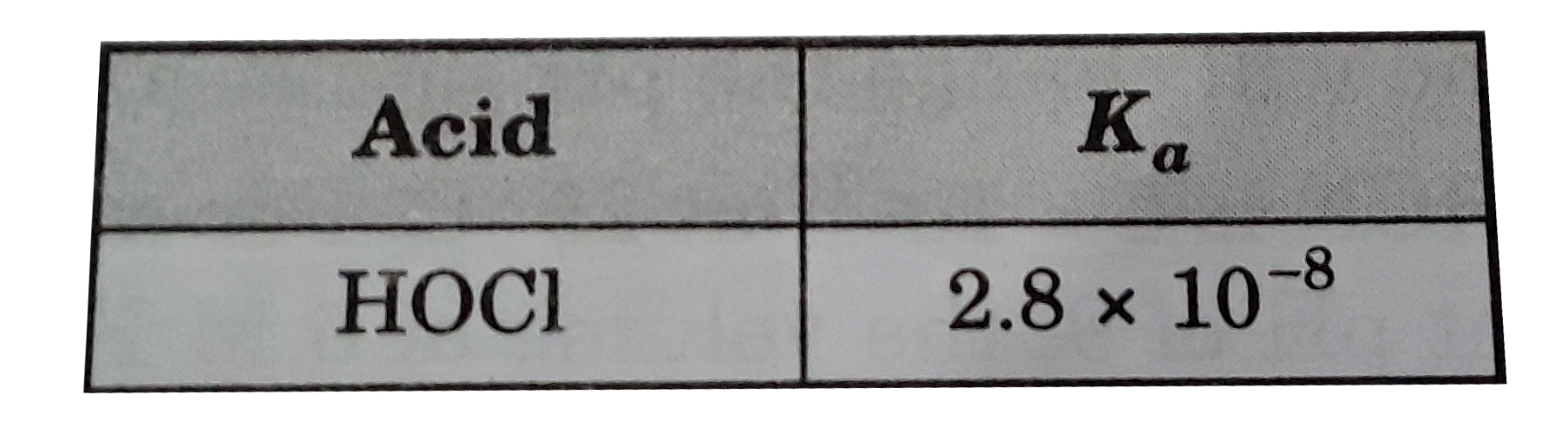
- What is the [OH^-] of a 0.65 M solution of NaOCl ?
Text Solution
|
- What is the [OH^-] of a 0.65 M solution of NaOCl ?
Text Solution
|
- The pH of 0.05 M Ba(OH)(2) solution is
Text Solution
|
- 0.050 M HCl के 20 mL को 0.1 M Ba(OH)(2) ke 30.0 mL के साथ मिलाने पर बन...
Text Solution
|
- सर्वसमिकाओ की सहायता से सरल कीजिये- 10 रु० का (0.65 xx 0.65 - 0.65 x...
Text Solution
|
- 0.05 M ammonium hydroxide solution is dissolved in 0.001 M ammonium ch...
Text Solution
|
- What is the [OH]^(-) in the final solution prepared by mixing 20.0mL o...
Text Solution
|
- 0.001 M Ba(OH)(2) विलयन का pH क्या होगा?
Text Solution
|
- 25 mL Ba(OH)(2) solution is neutralized by 35 mL 0.1 M HCl, what will ...
Text Solution
|