A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Textual Evaluation Solved(Write brief answer to the following questions.)|20 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise In Text Questions - Evaluate Yourself|8 VideosALKALI AND ALKALINE EARTH METALS
FULL MARKS|Exercise Additional Questions Solved|224 VideosBASIC CONCEPTS OF ORGANIC REACTIONS
FULL MARKS|Exercise Additional Questions Solved (5-Marks Question)|10 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS -Additional Questions Solved(5-Mark Questions)
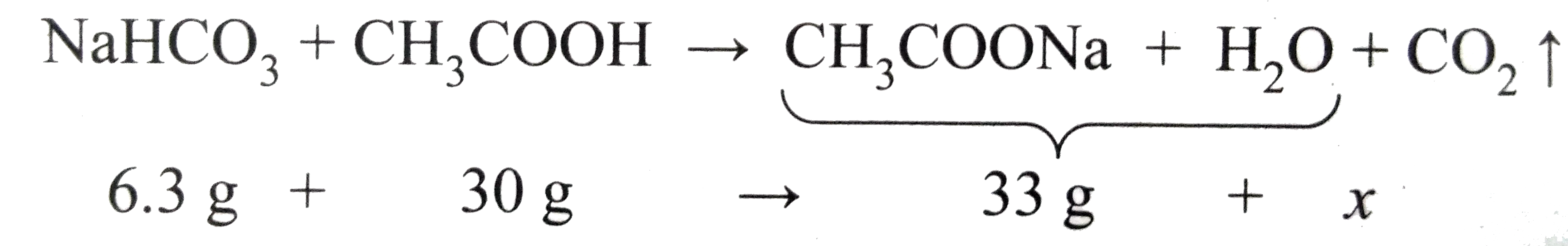
- When 6.3 g of sodium bicarbonate is added to 30 g of acetic acid solut...
Text Solution
|
- Define the following (a) equivalent mass of an acid (b) equivalent ma...
Text Solution
|
- Calculate the percentage composition of the elements present in lead n...
Text Solution
|
- Determine the empirical formula of a compound containing K=24.75%, Mn ...
Text Solution
|
- Write the steps to be followed for writing empirical formula.
Text Solution
|
- An organic compound was found to have contained carbon = 40.65%, hydro...
Text Solution
|
- A compound contains 32% carbon, 4% hydrogen and rest oxygen. Its vapou...
Text Solution
|
- Explain the different types of redox reactions with example.
Text Solution
|
- Write the steps to be followed while balancing redox equation by oxida...
Text Solution
|
- Balance the following equation by oxidation number method:
Text Solution
|
- Balance the following equation by ion-electron method.
Text Solution
|
- Define equivalent mass of an oxidising agent How would you calculat...
Text Solution
|
- Define equivalent mass of an reducing agent. How would you determin...
Text Solution
|
- A compound on analysis gave the following percentage composition: C = ...
Text Solution
|
- A laboratory analysis of an organic compound gives the following mass ...
Text Solution
|
- An insecticide has the following percentage composition by mass. 47.5 ...
Text Solution
|
- An organic fruit smelling compound on analysis has the following compo...
Text Solution
|
- Calculate the percentage composition of the elements present in magnes...
Text Solution
|
- Urea is prepared by the reaction between ammonia and carbon dioxide. ...
Text Solution
|
- Define oxidation number. What are the rules used to assign oxidation...
Text Solution
|
- Balance the following equation by oxidation number method C(6)H(6...
Text Solution
|