Text Solution
Verified by Experts
Topper's Solved these Questions
BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Textual Calculation based on Stoichiometry solved|6 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Additional Questions Solved(Choose the correct)|33 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Textual Evaluation Solved(Write brief answer to the following questions.)|20 VideosALKALI AND ALKALINE EARTH METALS
FULL MARKS|Exercise Additional Questions Solved|224 VideosBASIC CONCEPTS OF ORGANIC REACTIONS
FULL MARKS|Exercise Additional Questions Solved (5-Marks Question)|10 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS -In Text Questions - Evaluate Yourself
- By applying the knowledge of chemical classification, classify each of...
Text Solution
|
- Calculate the relative molecular mass of the following. (i) Ethano...
Text Solution
|
- Calculate the number of moles present in 9 g of ethane Calculate the...
Text Solution
|
- (a) 0.456 g of a metal gives 0.606 g of its chloride. Calculate the eq...
Text Solution
|
- A compound on analysis gave the following percentage composition: C - ...
Text Solution
|
- Experimental analysis of a compound containing the elements x,y,z on a...
Text Solution
|
- The balanced equation for a reaction is given below 2x + 3y rarr 4l + ...
Text Solution
|
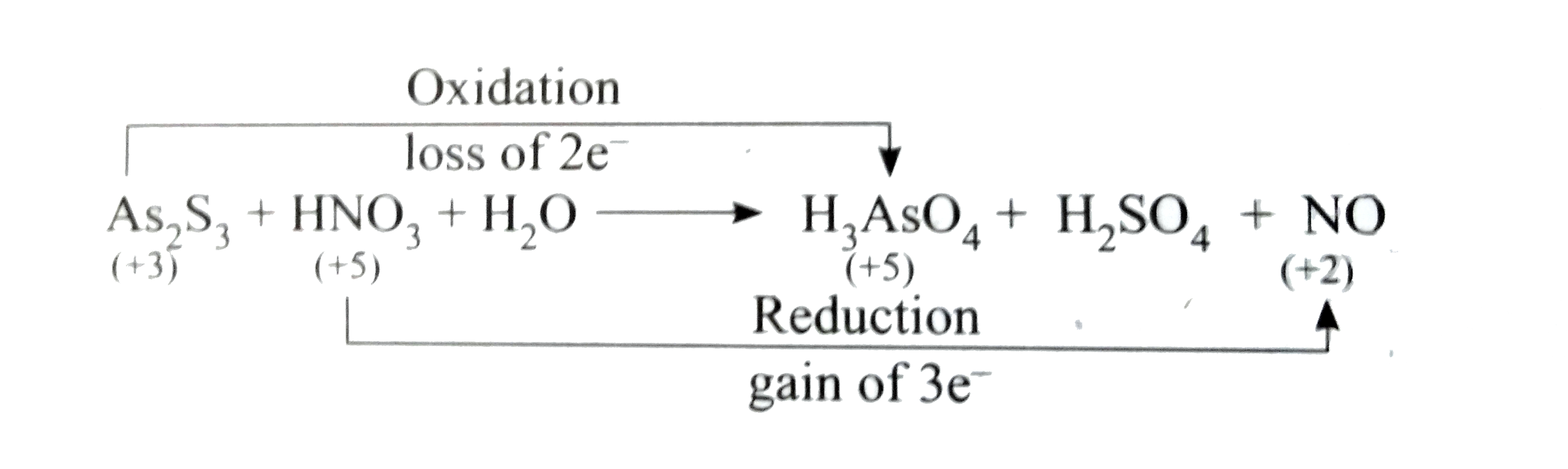
- Balance the following equation using oxidation number method As(2...
Text Solution
|