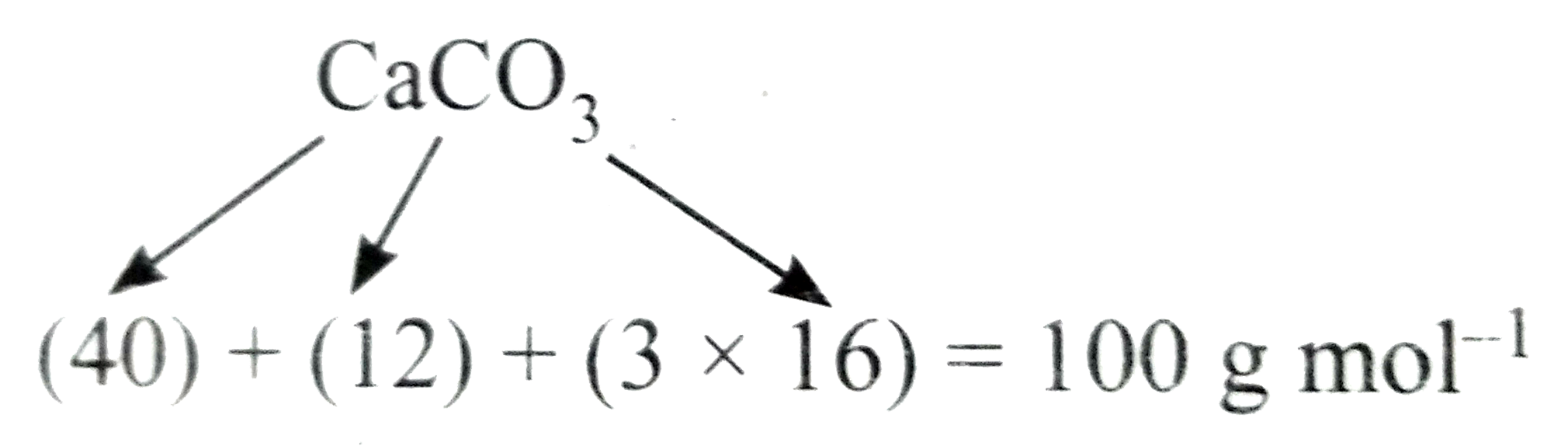Text Solution
Verified by Experts
Topper's Solved these Questions
BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Additional Questions Solved(Choose the correct)|33 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Additional Questions Solved(Match the following)|3 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise In Text Questions - Evaluate Yourself|8 VideosALKALI AND ALKALINE EARTH METALS
FULL MARKS|Exercise Additional Questions Solved|224 VideosBASIC CONCEPTS OF ORGANIC REACTIONS
FULL MARKS|Exercise Additional Questions Solved (5-Marks Question)|10 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS -Textual Calculation based on Stoichiometry solved
- How many moles of hydrogen is required to produce 10 moles of ammonia ...
Text Solution
|
- Calculate the amount of water produced by the combustion of 32 g of me...
Text Solution
|
- How much volume of carbon dioxide is produced when 50 g of calcium car...
Text Solution
|
- How much volume of chlorine is required to form 11.2 L of HC1 at 273 K...
Text Solution
|
- Calculate the percentage composition of the elements present in magnes...
Text Solution
|
- In a process, 646 g of ammonia is allowed to react with 1.144 kg of CO...
Text Solution
|