Text Solution
Verified by Experts
Topper's Solved these Questions
BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Additional Questions Solved(5-Mark Questions)|29 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Additional Questions Solved(2-Mark Questions)|41 VideosALKALI AND ALKALINE EARTH METALS
FULL MARKS|Exercise Additional Questions Solved|224 VideosBASIC CONCEPTS OF ORGANIC REACTIONS
FULL MARKS|Exercise Additional Questions Solved (5-Marks Question)|10 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS -Additional Questions Solved(3-Mark Questions)
- Explain about the classification of matter
Text Solution
|
- Calculate the mass of the following atoms in amu (a) Helium (mass...
Text Solution
|
- Calculate the number of atoms present in 1 Kg of gold.
Text Solution
|
- Calculate the molar volume of 146 g of HC1 gas and the number of molec...
Text Solution
|
- Calculate the molar mass of 20 L of gas weighing 23.2 g at STP.
Text Solution
|
- 0.6 g of a metal gives on oxidation 1 g of its oxide. Calculate its eq...
Text Solution
|
- How would you calculate the equivalent mass of anhydrous oxalic acid a...
Text Solution
|
- A compound on decomposition in the laboratory produces 24.5 g of nitro...
Text Solution
|
- What is the steps involve in the calculation of molecular formula from...
Text Solution
|
- What is combination reaction ? Give example.
Text Solution
|
- What is decomposition reaction? Give two examples
Text Solution
|
- What is displacement reactions ? Give its types. Explain with example.
Text Solution
|
- What is disproportionation reactions? Give example.
Text Solution
|
- What are competive electron transfer reaction ? Give example.
Text Solution
|
- Balance the following equation using oxidation number method. S +...
Text Solution
|
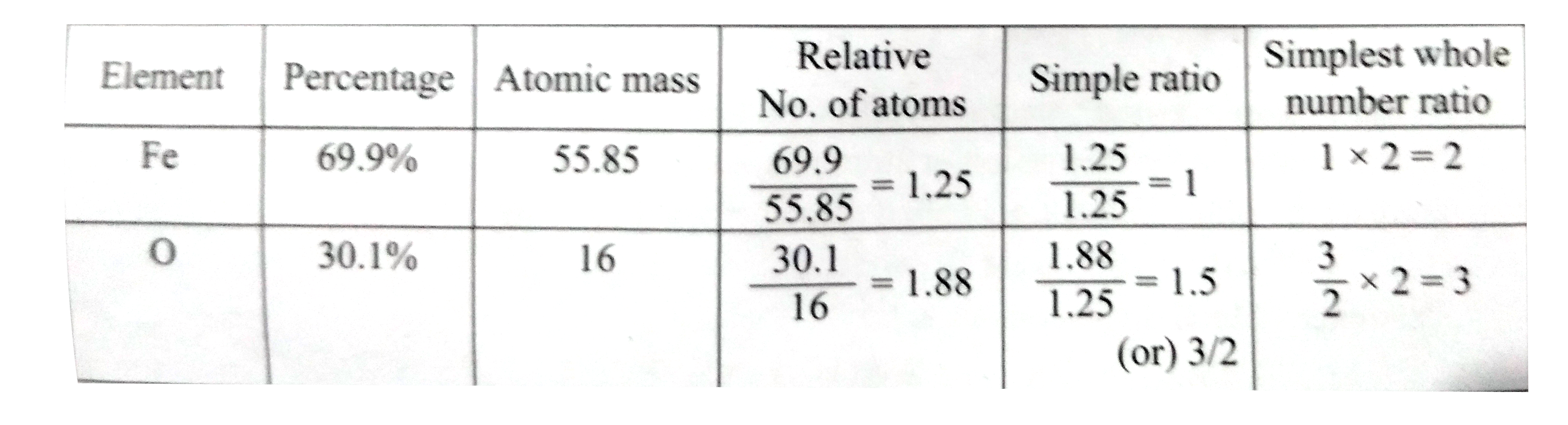
- Determine the empirical formula of an oxide of iron which has 69.9% ir...
Text Solution
|
- In three moles of ethane (C(2)H(6)) calculate the following: (i) ...
Text Solution
|
- Chlorine is prepared in the laboratory by treating manganese dioxide (...
Text Solution
|
- The density of Water at room temperature is 1.0 g/ml. How many molecul...
Text Solution
|
- Balance the following equation by oxidation number method. MnO(4)...
Text Solution
|