Text Solution
Verified by Experts
Topper's Solved these Questions
BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
FULL MARKS|Exercise Additional Questions Solved(3-Mark Questions)|27 VideosALKALI AND ALKALINE EARTH METALS
FULL MARKS|Exercise Additional Questions Solved|224 VideosBASIC CONCEPTS OF ORGANIC REACTIONS
FULL MARKS|Exercise Additional Questions Solved (5-Marks Question)|10 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS -Additional Questions Solved(5-Mark Questions)
- Balance the following equation by ion-electron method.
Text Solution
|
- Define equivalent mass of an oxidising agent How would you calculat...
Text Solution
|
- Define equivalent mass of an reducing agent. How would you determin...
Text Solution
|
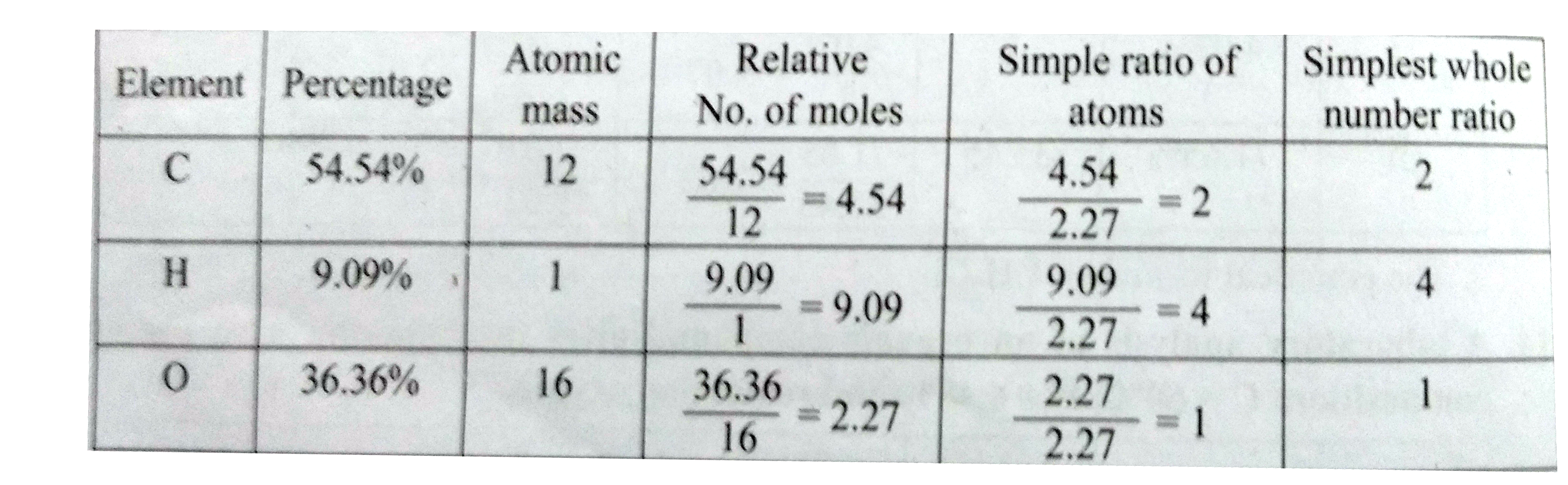
- A compound on analysis gave the following percentage composition: C = ...
Text Solution
|
- A laboratory analysis of an organic compound gives the following mass ...
Text Solution
|
- An insecticide has the following percentage composition by mass. 47.5 ...
Text Solution
|
- An organic fruit smelling compound on analysis has the following compo...
Text Solution
|
- Calculate the percentage composition of the elements present in magnes...
Text Solution
|
- Urea is prepared by the reaction between ammonia and carbon dioxide. ...
Text Solution
|
- Define oxidation number. What are the rules used to assign oxidation...
Text Solution
|
- Balance the following equation by oxidation number method C(6)H(6...
Text Solution
|
- Balance the following equation by oxidation number method. KMNO(4...
Text Solution
|
- Balance the following equation by oxidation number method. KMnO(4...
Text Solution
|
- Balancing of molecular equation in alkaline medium. MnO(2)+O(2)+K...
Text Solution
|
- Explain the steps involved in ion-electron method for balancing redox ...
Text Solution
|
- Write balanced equation for the oxidation of Ferrous ions to Ferric io...
Text Solution
|
- A flask A contains 0.5 mole of oxygen gas. Another flask B contains 0....
Text Solution
|
- Formulate possible compounds of ‘Cl’ in its oxidation state is: 0...
Text Solution
|
- The Mn^(3+) ion is unstable in solution and undergoes disproportionati...
Text Solution
|
- Chlorine is used to purify drinking water. Excess of chlorine is harmf...
Text Solution
|