Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(M.C.Q)|38 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(Match the follwing)|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Textual Evaluation Solved( II brief question)|24 VideosHYDROGEN
FULL MARKS|Exercise Additional Questions Solved 5-Mark Questions|10 VideosPHYSICAL AND CHEMICAL EQUILIBRIUM
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)|3 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-PERIODIC CLASSIFICATION OF ELEMENTS -In-Text Question-Evaluate Yourself
- What is the basic difference in approach between Mendeleev's periodic ...
Text Solution
|
- The element with atomic number 120 has not been discovered so far. Wha...
Text Solution
|
- Predict the position of the element in periodic table satisfying the e...
Text Solution
|
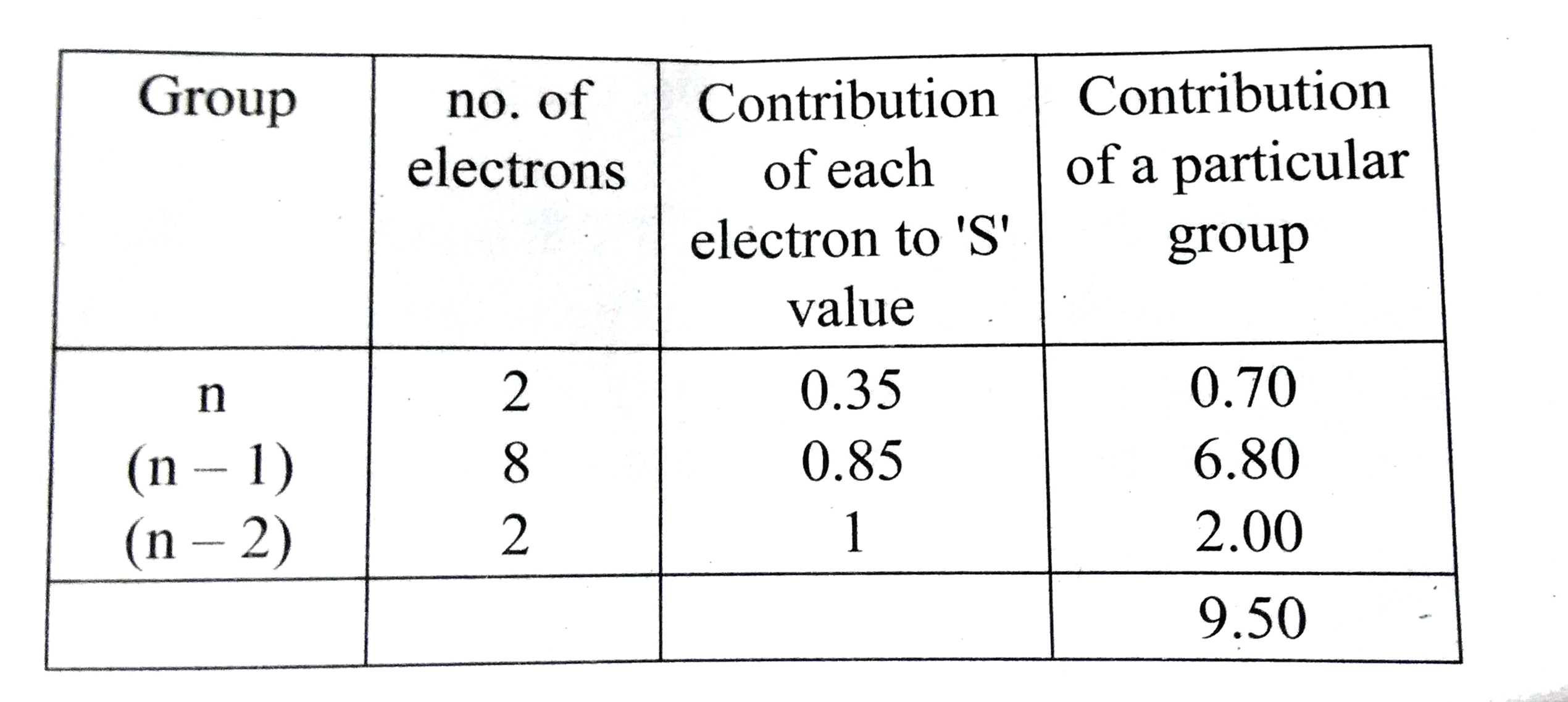
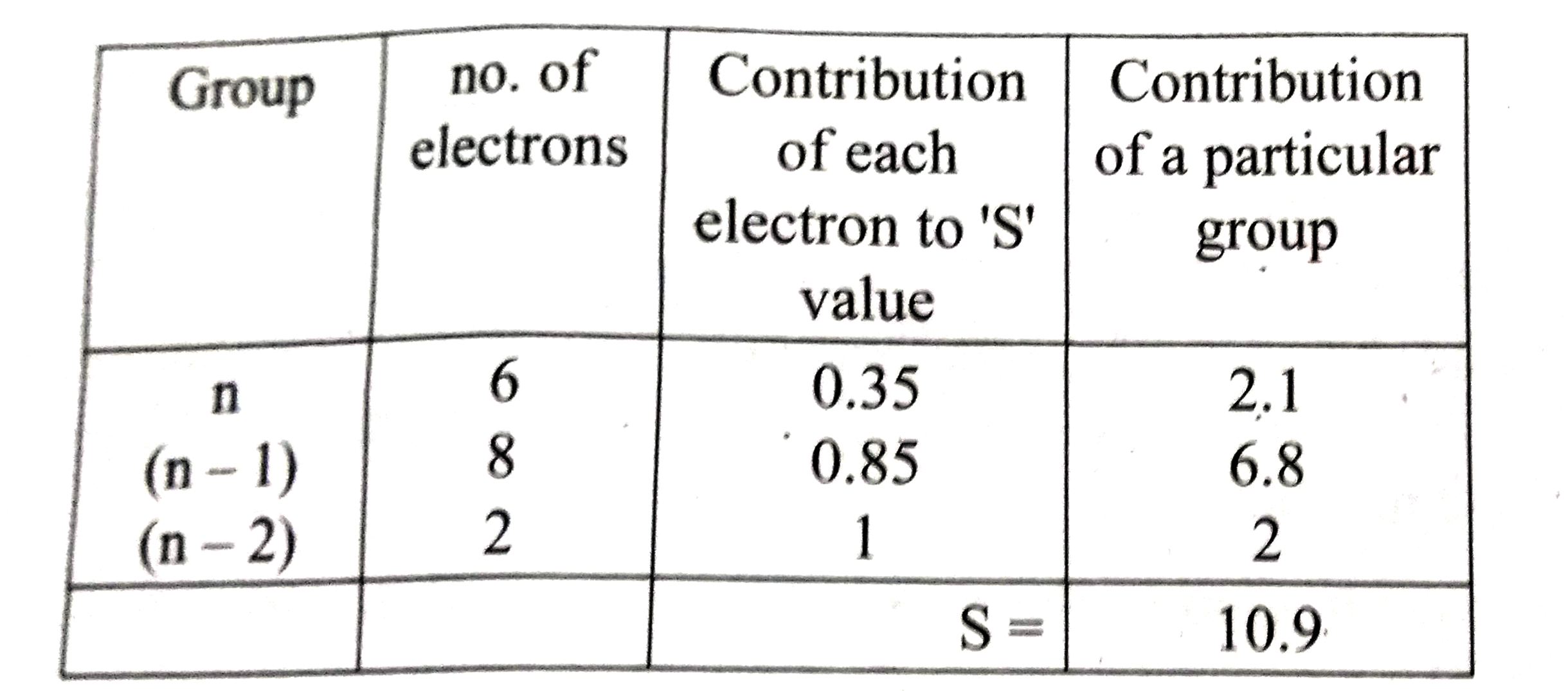
- Using Slater's rule calculate the effective nuclear charge on a 3p ele...
Text Solution
|
- A student reported the ionic radii of isocelectronic species X^(3+), Y...
Text Solution
|
- The first ionisation energy (IE1) and second ionisation energy (IE2) o...
Text Solution
|
- The electron gain enthalpy of chlorine is 348 kJ mol^(-1). How much en...
Text Solution
|