Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-THERMODYNAMICS -Textual Question Solved
- Define Gibb's free energy .
Text Solution
|
- Calculate the work done when 2 moles of an ideal gas expands reversibl...
Text Solution
|
- In a constant volume calorimeter, 3.5 g of a gas with molecular weight...
Text Solution
|
- Calculate the entropy change in the system and surroundings, and the t...
Text Solution
|
- 1 mole of an ideal gas, maintained at 4.1 atm and at a certain tempera...
Text Solution
|
- 30.4 kJ is required to melt one mole of sodium chloride. The entropy c...
Text Solution
|
- Calculate the standard heat of formation of propane, if its heat of co...
Text Solution
|
- You are given normal boiling points and standard enthalpies of vaporiz...
Text Solution
|
- DeltaH and DeltaS for the reaction Ag(2)O((s))rarr2Ag((s))+1/2O(2((g...
Text Solution
|
- What is the equilibrium constant K(eq) for the following reaction at 4...
Text Solution
|
- Cyanamide (NH2CN) is completely burnt in excess oxygen in a bomb calor...
Text Solution
|
- Calculate the enthalpy of hydrogenation of ethylene from the following...
Text Solution
|
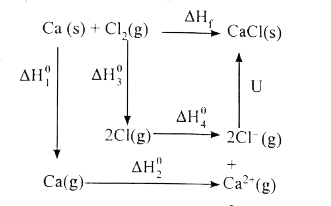
- Calculate the lattice energy of CaCl2 from the given data. Ca((s)) +...
Text Solution
|
- Calculate the enthalpy change for the reaction Fe2O3 +3CO to 2Fe +3CO2...
Text Solution
|
- When 1-pentyne (A) is treated with 4N alcoholic KOH at 175^@C, it is c...
Text Solution
|
- At 33K , N(2)O(4) is fifty percent dissociated Calculate the standard ...
Text Solution
|
- The standard enthalpies of formation of SO2 and SO3 are -297 "kJ mol"...
Text Solution
|
- For the reaction at 298 K : 2A +B to C DeltaH="400 KJ mol"^(-1) , De...
Text Solution
|
- Find out the value of equilibrium constant for the following reaction ...
Text Solution
|
- A gas mixture of 3.67 lit of ethylene and methane on complete combusti...
Text Solution
|