Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-THERMODYNAMICS -In-Text Questions -Evaluate Yourself
- Calculate DeltaHf^@ for the reaction CO2(g) +H2(g) to CO(g) + H2O(g) g...
Text Solution
|
- Calculate the amount of heat necessary to raise 180 g of water from 25...
Text Solution
|
- From the following data at constant volume for combustion of benzene, ...
Text Solution
|
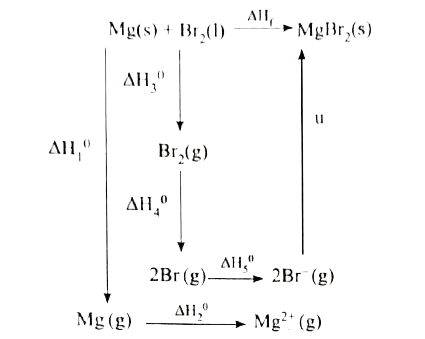
- When a mole of magnesium bromide is prepared from 1 mole of magnesium ...
Text Solution
|
- An engine operating between 127^@C and 47^@C takes some specified amou...
Text Solution
|
- Urea on hydrolysis produces ammonia and carbon dioxide. The standard e...
Text Solution
|
- Calculate the entropy change when 1 mole of ethanol is evaporated at 3...
Text Solution
|
- For a chemical reaction the values of DeltaH and DeltaS at 300 K are -...
Text Solution
|