Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-THERMODYNAMICS -Additional Questions Solved (5-Mark Questions)
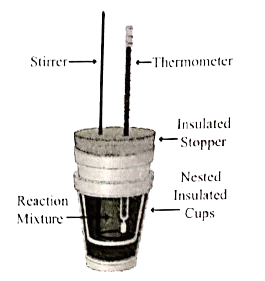
- Explain how heat absorbed at constant pressure is measured using coff...
Text Solution
|
- List the characteristics of entropy.
Text Solution
|
- Explain about the characteristics of work.
Text Solution
|
- Write the relationship between Permutation and Combination?
Text Solution
|
- Write the various definition of first law of thermodynamics.
Text Solution
|
- Derive the various mathematical statements of the first law.
Text Solution
|
- What are the characteristics of enthalpy?
Text Solution
|
- What are thermochemical equation? What are the conventions adopted in ...
Text Solution
|
- Calculate the values of DeltaU and DeltaH for an ideal gas in terms of...
Text Solution
|
- Suggest and explain an indirect method to calculate lattice enthalpy o...
Text Solution
|
- The standard free energy change (DeltaG^0) is related to equilibrium c...
Text Solution
|