Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED - 5 MARKS QUESTIONS|8 VideosSOLUTIONS
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED - NUMERICAL PROBLEMS|15 VideosSOLUTIONS
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED - 2 MARKS QUESTIONS|38 VideosSAMPLE PAPER-8
FULL MARKS|Exercise Part-IV|10 VideosSOLVED PAPER 03
FULL MARKS|Exercise PART-IV|10 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-SOLUTIONS -ADDITIONAL QUESTIONS SOLVED - 3 MARKS QUESTIONS
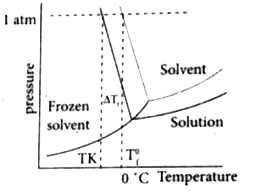
- Graphically prove that T(b) is greater than T(b) ^(@).
Text Solution
|
- Derive the relationship between the elevation of boiling point and mol...
Text Solution
|
- Write short note an freezing point depression in freezing point and cr...
Text Solution
|
- Define (i) cryoscopic constant (ii) ebullioscopic constant
Text Solution
|
- Discuss the significances of osmotic-pressure over other colligative p...
Text Solution
|
- What is haemolysis? Why intravenous fluid are isotonic to blood ?
Text Solution
|
- What is reverse osmosis?
Text Solution
|
- Explain about the application of reverse osmosis in water purification...
Text Solution
|
- Acetic acid is found to have molar mass as 120 g mol ^(-1). Prove it.
Text Solution
|
- Prove that the depression in freezing point is a colligative property.
Text Solution
|
- The estimated van't Hoff factor for acetic acid solution in benzene is...
Text Solution
|
- Distinguish between ideal and non-ideal solution.
Text Solution
|
- The function f(x) = {{:(2, x lt=1),(x , x gt 1 ):} is not different...
Text Solution
|
- State Henry's law and mention some of its important applications.
Text Solution
|
- What type of non-idealities are exhibited by cyclohexane-ethanol and a...
Text Solution
|
- Given below is the sketch of a plant for carrying out a process. ...
Text Solution
|
- Define the term osmotic pressure. Describe how the molecular mass of a...
Text Solution
|
- (a) Menthol is a crystalline substance with peppermint taste. A.6.2% s...
Text Solution
|
- The specific heat of water is
Text Solution
|
- State Henry's law for slubility of a gas in a liquid. Explain the sign...
Text Solution
|