Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SOLUTIONS -ADDITIONAL QUESTIONS SOLVED - 5 MARKS QUESTIONS
- (i) Define solution. (ii) Explain the types of solutions with suitab...
Text Solution
|
- (i) Define Solubility (ii) Explain about the factors that influences...
Text Solution
|
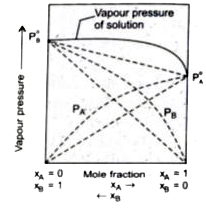
- Which of the following shows positive deviation from Raoult's law ?
Text Solution
|
- How would you determine molar mass from relative lowering of vapour pr...
Text Solution
|
- How would you determine molar mass from relative lowering of vapour pr...
Text Solution
|
- What are non-ideal solution ? Give example.
Text Solution
|
- Which of the following shows positive deviation from Raoult's law ?
Text Solution
|
- Calculate the gram molecular mass of the following NH(3)
Text Solution
|