Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (CHOOSE THE CORRECT ANSWER.)|50 VideosCHEMICAL BONDING
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (MATCH THE FOLLOWING.)|11 VideosCHEMICAL BONDING
FULL MARKS|Exercise TEXTUAL EVALUATION SOLVED (SHORT ANSWER QUESTIONS.)|25 VideosBASIC CONCEPTS OF ORGANIC REACTIONS
FULL MARKS|Exercise Additional Questions Solved (5-Marks Question)|10 VideosENVIRONMENTAL CHEMISTRY
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED ( 5-Mark Questions )|8 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-CHEMICAL BONDING -IN TEXT QUESTION-EVALUATE YOURSELF
- Draw the lewis structures for (i) Nitrous acid (HN0(2)) (ii) Phospho...
Text Solution
|
- Calculate the formal charge on each atom of carbonyl chloride (COCl(2)...
Text Solution
|
- Explain the ionic bond formation in MgO and CaF(2) :
Text Solution
|
- Write the resonance structures for (i) Ozone molecule (ii) N(2)O
Text Solution
|
- Of the two molecules OCS and CS(2) which one has higher dipole moment ...
Text Solution
|
- Arrange the following in the decreasing order of Bond angle (i) CH(4...
Text Solution
|
- Bond angle in PH(4)^(+) is higher than in PH(3). Why?
Text Solution
|
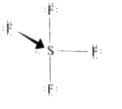
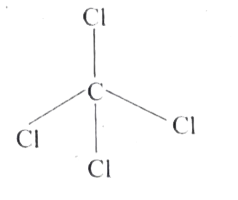
- Explain the bond formation in SF(4) and CCl(4)' using hybridisation c...
Text Solution
|
- The observed bond length of N(2)^(+) is larger than N(2) while the bon...
Text Solution
|
- Draw the MO diagram for acetylide ion C(2)^(2-) and calculate its bond...
Text Solution
|