Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (5-MARK QUESTIONS)|14 VideosCHEMICAL BONDING
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (2-MARK QUESTIONS)|40 VideosBASIC CONCEPTS OF ORGANIC REACTIONS
FULL MARKS|Exercise Additional Questions Solved (5-Marks Question)|10 VideosENVIRONMENTAL CHEMISTRY
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED ( 5-Mark Questions )|8 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-CHEMICAL BONDING -ADDITIONAL QUESTIONS SOLVED (3-MARK QUESTIONS)
- Draw the structure of AB(2), AB(3), AB(3)L type of molecules with exam...
Text Solution
|
- Give example and structure of (i) AB(3)L (ii) AB(5) (iii) AB(2)L(2) ...
Text Solution
|
- Draw the shape of (i) XeF(2) (ii) IOF(5) (iii) XeOF(4)
Text Solution
|
- Explain the bonding in oxygen molecule.
Text Solution
|
- Explain about the molecular orbital diagram of hydrogen molecule.
Text Solution
|
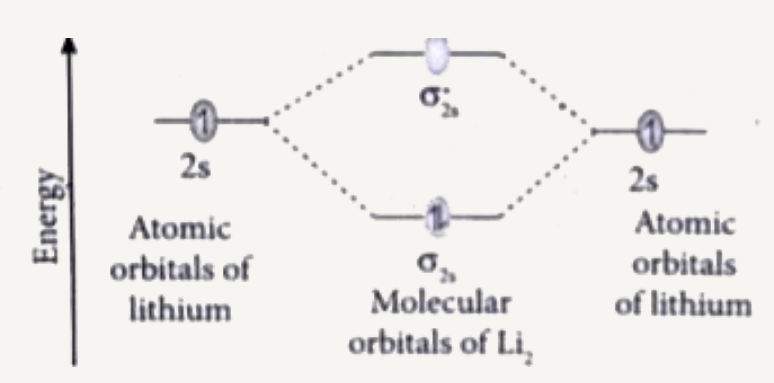
- Draw and explain the M.O. diagram of lithium molecule.
Text Solution
|
- Draw and explain the M.O. diagram of Boron molecule.
Text Solution
|
- Draw and explain the molecular orbital diagram of carbon molecule.
Text Solution
|
- Write Lewis dot symbols for atoms of the following elements: Mg, Na, B...
Text Solution
|
- Write Lewis symbols for the following atoms and ions: S and S^(2-) , A...
Text Solution
|
- Draw the Lewis structures for the following molecules and ions: H(2)...
Text Solution
|
- Define Octet rule. Write its significance and limitations.
Text Solution
|
- Write the resonánce structure for SO(3), NO(2) and NO(3)^(-)
Text Solution
|
- What do you understand by bond pairs and lóne pairs of electrons? Illu...
Text Solution
|
- Distinguish sigma and pi - bonds.
Text Solution
|
- Write the important conditions required for the linear combination of ...
Text Solution
|
- What are Lewis structures? Write the Lewis structure of H(2), BeF(2) a...
Text Solution
|
- What are the main postulates of Valence Shell Electron Pair Repulsion ...
Text Solution
|
- Apart from tetrahedral geometry, another possible geometry for CH(4) i...
Text Solution
|
- Explain why BeH(2) molecule has a zero dipole moment although the Be -...
Text Solution
|